当前位置:
X-MOL 学术
›
Nat. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Design of aqueous redox-enhanced electrochemical capacitors with high specific energies and slow self-discharge.
Nature Communications ( IF 14.7 ) Pub Date : 2015-Aug-04 , DOI: 10.1038/ncomms8818 Sang-Eun Chun , Brian Evanko , Xingfeng Wang , David Vonlanthen , Xiulei Ji , Galen D. Stucky , Shannon W. Boettcher
Nature Communications ( IF 14.7 ) Pub Date : 2015-Aug-04 , DOI: 10.1038/ncomms8818 Sang-Eun Chun , Brian Evanko , Xingfeng Wang , David Vonlanthen , Xiulei Ji , Galen D. Stucky , Shannon W. Boettcher
|
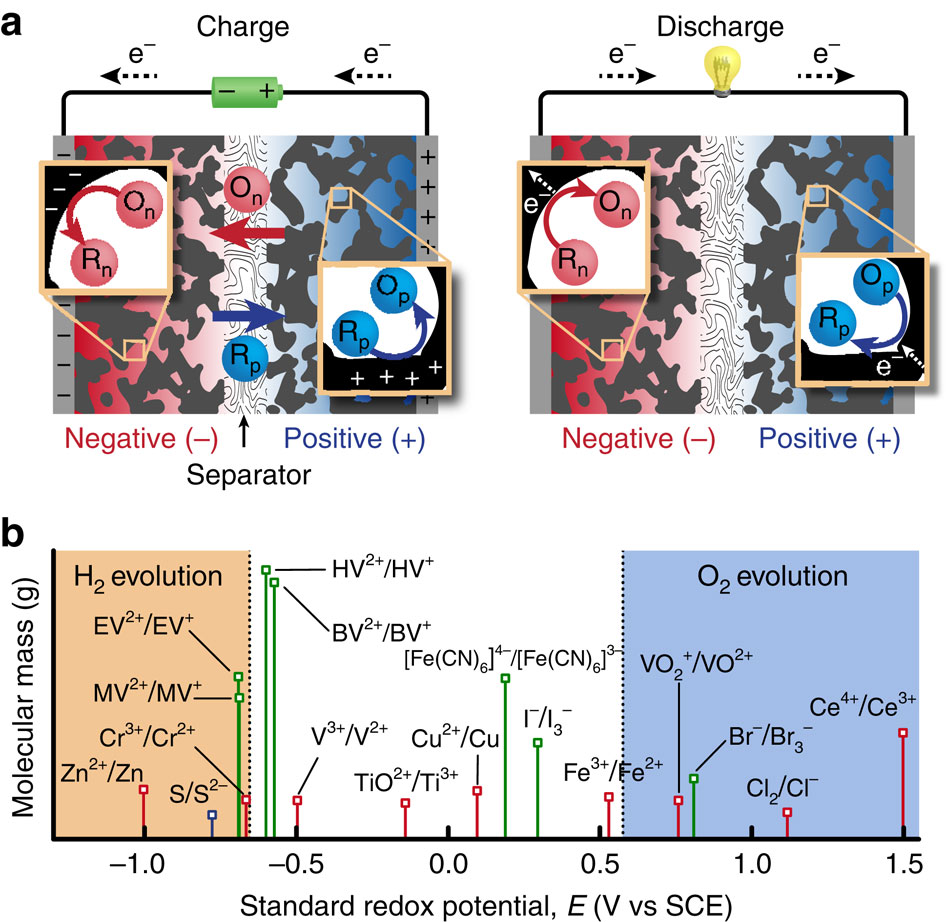
Electrochemical double-layer capacitors exhibit high power and long cycle life but have low specific energy compared with batteries, limiting applications. Redox-enhanced capacitors increase specific energy by using redox-active electrolytes that are oxidized at the positive electrode and reduced at the negative electrode during charging. Here we report characteristics of several redox electrolytes to illustrate operational/self-discharge mechanisms and the design rules for high performance. We discover a methyl viologen (MV)/bromide electrolyte that delivers a high specific energy of ∼14 Wh kg(-1) based on the mass of electrodes and electrolyte, without the use of an ion-selective membrane separator. Substituting heptyl viologen for MV increases stability, with no degradation over 20,000 cycles. Self-discharge is low, due to adsorption of the redox couples in the charged state to the activated carbon, and comparable to cells with inert electrolyte. An electrochemical model reproduces experiments and predicts that 30-50 Wh kg(-1) is possible with optimization.
中文翻译:

具有高比能和缓慢自放电的水性氧化还原增强型电化学电容器的设计。
电化学双层电容器具有高功率和长循环寿命,但与电池相比具有较低的比能,从而限制了应用。氧化还原增强型电容器通过使用氧化还原活性电解质来提高比能,该电解质在充电过程中在正极被氧化,在负极被还原。在这里,我们报告几种氧化还原电解质的特性,以说明操作/自放电机理和高性能设计规则。我们发现基于电极和电解质的质量,无需使用离子选择膜分离器即可产生约14 Wh kg(-1)的高比能的甲基紫精(MV)/溴化物电解质。用庚基紫精代替MV可提高稳定性,在20,000个循环内不会降解。自放电率低 由于氧化还原电偶在带电状态下吸附到活性炭上,与具有惰性电解质的电池相当。电化学模型可重现实验,并预测优化后可能产生30-50 Wh kg(-1)。
更新日期:2015-08-06
中文翻译:

具有高比能和缓慢自放电的水性氧化还原增强型电化学电容器的设计。
电化学双层电容器具有高功率和长循环寿命,但与电池相比具有较低的比能,从而限制了应用。氧化还原增强型电容器通过使用氧化还原活性电解质来提高比能,该电解质在充电过程中在正极被氧化,在负极被还原。在这里,我们报告几种氧化还原电解质的特性,以说明操作/自放电机理和高性能设计规则。我们发现基于电极和电解质的质量,无需使用离子选择膜分离器即可产生约14 Wh kg(-1)的高比能的甲基紫精(MV)/溴化物电解质。用庚基紫精代替MV可提高稳定性,在20,000个循环内不会降解。自放电率低 由于氧化还原电偶在带电状态下吸附到活性炭上,与具有惰性电解质的电池相当。电化学模型可重现实验,并预测优化后可能产生30-50 Wh kg(-1)。















































 京公网安备 11010802027423号
京公网安备 11010802027423号