当前位置:
X-MOL 学术
›
Chem. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Li2Cu2O(SO4)2: a Possible Electrode for Sustainable Li-Based Batteries Showing a 4.7 V Redox Activity vs Li+/Li0
Chemistry of Materials ( IF 7.2 ) Pub Date : 2015-04-07 00:00:00 , DOI: 10.1021/acs.chemmater.5b00588
Meiling Sun 1 , Gwenaëlle Rousse 1, 2, 3 , Artem M. Abakumov 4 , Matthieu Saubanère 3, 5 , Marie-Liesse Doublet 3, 5 , Juan Rodríguez-Carvajal 6 , Gustaaf Van Tendeloo 4 , Jean-Marie Tarascon 1, 2, 3
Chemistry of Materials ( IF 7.2 ) Pub Date : 2015-04-07 00:00:00 , DOI: 10.1021/acs.chemmater.5b00588
Meiling Sun 1 , Gwenaëlle Rousse 1, 2, 3 , Artem M. Abakumov 4 , Matthieu Saubanère 3, 5 , Marie-Liesse Doublet 3, 5 , Juan Rodríguez-Carvajal 6 , Gustaaf Van Tendeloo 4 , Jean-Marie Tarascon 1, 2, 3
Affiliation
|
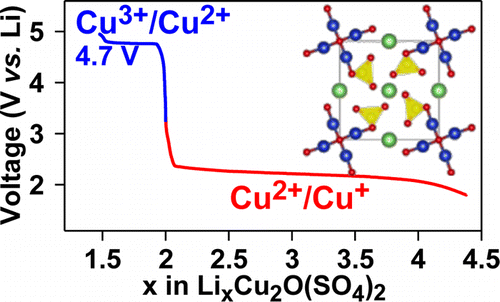
Li-ion batteries rely on the use of insertion positive electrodes with performances scaling with the redox potential of the 3D metals accompanying Li-uptake/removal. Although not commonly studied, the Cu2+/Cu3+ redox potential has been predicted from theoretical calculations to possibly offer a high operating voltage redox couple. We herein report the synthesis and crystal structure of a hitherto-unknown oxysulfate phase, Li2Cu2O(SO4)2, which contains infinite edge-sharing CuO4 chains and presents attractive electrochemical redox activity with respect to Li+/Li, namely amphoteric characteristics. Li2Cu2O(SO4)2 shows redox activity at 4.7 V vs Li+/Li0 corresponding to the oxidation of Cu2+ to Cu3+ enlisting ligand holes and associated with the reversible uptake-removal of 0.3 Li. Upon reduction, this compound reversibly uptakes ∼2 Li at an average potential of about 2.5 V vs Li+/Li0, associated with the Cu2+/Cu+ redox couple. The mechanism of the reactivity upon reduction is discussed in detail, with particular attention to the occasional appearance of an oscillation wave in the discharge profile. Our work demonstrates that Cu-based compounds can indeed be fertile scientific ground in the search for new high-energy-density electrodes.
中文翻译:

Li 2 Cu 2 O(SO 4)2:一种可持续的基于Li的电池的电极,其对Li + / Li 0的氧化还原活性为4.7 V
锂离子电池依赖于插入式正极的使用,其性能随伴随着锂吸收/去除的3D金属的氧化还原电势而变化。尽管尚未进行广泛研究,但已从理论计算中预测出了Cu 2+ / Cu 3+氧化还原电势,可能会提供较高的工作电压氧化还原电对。我们在此报告了迄今未知的硫酸硫酸盐相Li 2 Cu 2 O(SO 4)2的合成和晶体结构,该相含有无限的边沿共享CuO 4链,并且相对于Li + / Li具有诱人的电化学氧化还原活性,即两性特征。锂2铜2 O(SO 4)2示出了在4.7V,相对于Li氧化还原活性的+ /锂0对应于Cu的氧化2+成Cu 3+争取配体的孔和与可逆摄取去除0.3立相关联。还原后,该化合物以约2.5 V的平均电势相对于Li + / Li 0可逆地摄取〜2 Li,这与Cu 2+ / Cu +有关氧化还原夫妇。详细讨论了还原时反应性的机理,尤其要注意在放电曲线中偶尔会出现振荡波。我们的工作表明,铜基化合物确实可以成为寻找新的高能量密度电极的沃土科学依据。
更新日期:2015-04-07
中文翻译:

Li 2 Cu 2 O(SO 4)2:一种可持续的基于Li的电池的电极,其对Li + / Li 0的氧化还原活性为4.7 V
锂离子电池依赖于插入式正极的使用,其性能随伴随着锂吸收/去除的3D金属的氧化还原电势而变化。尽管尚未进行广泛研究,但已从理论计算中预测出了Cu 2+ / Cu 3+氧化还原电势,可能会提供较高的工作电压氧化还原电对。我们在此报告了迄今未知的硫酸硫酸盐相Li 2 Cu 2 O(SO 4)2的合成和晶体结构,该相含有无限的边沿共享CuO 4链,并且相对于Li + / Li具有诱人的电化学氧化还原活性,即两性特征。锂2铜2 O(SO 4)2示出了在4.7V,相对于Li氧化还原活性的+ /锂0对应于Cu的氧化2+成Cu 3+争取配体的孔和与可逆摄取去除0.3立相关联。还原后,该化合物以约2.5 V的平均电势相对于Li + / Li 0可逆地摄取〜2 Li,这与Cu 2+ / Cu +有关氧化还原夫妇。详细讨论了还原时反应性的机理,尤其要注意在放电曲线中偶尔会出现振荡波。我们的工作表明,铜基化合物确实可以成为寻找新的高能量密度电极的沃土科学依据。

































 京公网安备 11010802027423号
京公网安备 11010802027423号