当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Transition States and Control of Substrate Preference in the Promiscuous Phosphatase PP1
Biochemistry ( IF 2.9 ) Pub Date : 2017-07-21 00:00:00 , DOI: 10.1021/acs.biochem.7b00441
Yuan Chu 1 , Nicholas H. Williams 2 , Alvan C. Hengge 1
Biochemistry ( IF 2.9 ) Pub Date : 2017-07-21 00:00:00 , DOI: 10.1021/acs.biochem.7b00441
Yuan Chu 1 , Nicholas H. Williams 2 , Alvan C. Hengge 1
Affiliation
|
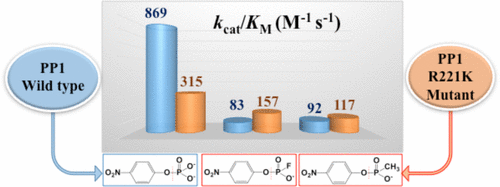
Catalytically promiscuous enzymes are an attractive frontier for biochemistry, because enzyme promiscuities not only plausibly explain enzyme evolution through the mechanism of gene duplication but also could provide an efficient route to changing the catalytic function of proteins by mimicking this evolutionary process. PP1γ is an effectively promiscuous phosphatase for the hydrolysis of both monoanionic and dianionic phosphate ester-based substrates. In addition to its native phosphate monoester substrate, PP1γ catalyzes the hydrolysis of aryl methylphosphonates, fluorophosphate esters, phosphorothioate esters, and phosphodiesters, with second-order rate accelerations that fall within the narrow range of 1011–1013. In contrast to the different transition states in the uncatalyzed hydrolysis reactions of these substrates, PP1γ catalyzes their hydrolysis through similar transition states. PP1γ does not catalyze the hydrolysis of a sulfate ester, which is unexpected. The PP1γ active site is tolerant of variations in the geometry of bound ligands, which permit the effective catalysis even of substrates whose steric requirements may result in perturbations to the positioning of the transferring group, both in the initial enzyme–substrate complex and in the transition state. The conservative mutation of arginine 221 to lysine results in a mutant that is a more effective catalyst toward monoanionic substrates. The surprising conversion of substrate preference lends support to the notion that mutations following gene duplication can result in an altered enzyme with different catalytic capabilities and preferences and may provide a pathway for the evolution of new enzymes.
中文翻译:

混杂态磷酸酶PP1中的过渡态和底物偏好的控制。
催化混杂酶是生物化学的一个有吸引力的前沿领域,因为酶混杂不仅可以通过基因复制机制合理地解释酶的进化,而且可以通过模仿这种进化过程来提供改变蛋白质催化功能的有效途径。PP1γ是一种有效的混杂磷酸酶,可水解基于单阴离子和双阴离子磷酸酯的底物。除了其天然的磷酸单酯底物外,PP1γ还催化芳基甲基膦酸酯,氟代磷酸酯,硫代磷酸酯和磷酸二酯的水解,其二级速率加速范围在10 11 –10 13的狭窄范围内。。与这些底物的未催化水解反应中的不同过渡态相反,PP1γ通过相似的过渡态催化其水解。PP1γ不会催化硫酸酯的水解,这是出乎意料的。PP1γ活性位点可耐受结合的配体的几何形状变化,即使在初始酶-底物复合物和过渡态中,其空间要求可能会干扰转移基团定位的底物也可有效催化状态。精氨酸221向赖氨酸的保守突变导致突变体,其是对单阴离子底物更有效的催化剂。
更新日期:2017-07-22
中文翻译:

混杂态磷酸酶PP1中的过渡态和底物偏好的控制。
催化混杂酶是生物化学的一个有吸引力的前沿领域,因为酶混杂不仅可以通过基因复制机制合理地解释酶的进化,而且可以通过模仿这种进化过程来提供改变蛋白质催化功能的有效途径。PP1γ是一种有效的混杂磷酸酶,可水解基于单阴离子和双阴离子磷酸酯的底物。除了其天然的磷酸单酯底物外,PP1γ还催化芳基甲基膦酸酯,氟代磷酸酯,硫代磷酸酯和磷酸二酯的水解,其二级速率加速范围在10 11 –10 13的狭窄范围内。。与这些底物的未催化水解反应中的不同过渡态相反,PP1γ通过相似的过渡态催化其水解。PP1γ不会催化硫酸酯的水解,这是出乎意料的。PP1γ活性位点可耐受结合的配体的几何形状变化,即使在初始酶-底物复合物和过渡态中,其空间要求可能会干扰转移基团定位的底物也可有效催化状态。精氨酸221向赖氨酸的保守突变导致突变体,其是对单阴离子底物更有效的催化剂。

































 京公网安备 11010802027423号
京公网安备 11010802027423号