当前位置:
X-MOL 学术
›
J. Phys. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Salt Promotes Protonation of Amine Groups at Air/Water Interface
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2017-07-21 00:00:00 , DOI: 10.1021/acs.jpclett.7b01198 Woongmo Sung 1 , Zaure Avazbaeva 1 , Doseok Kim 1
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2017-07-21 00:00:00 , DOI: 10.1021/acs.jpclett.7b01198 Woongmo Sung 1 , Zaure Avazbaeva 1 , Doseok Kim 1
Affiliation
|
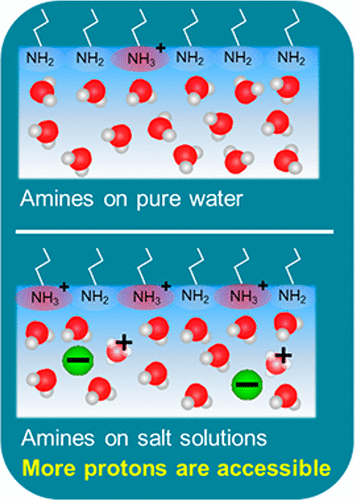
Interfacial water reorientation caused by charged Langmuir monolayers consisting of primary fatty amine (ODA) and cationic lipid having quaternary amine headgroup (DPTAP) were investigated by interface-selective vibrational sum-frequency generation spectroscopy. For DPTAP monolayer, initially large sum-frequency intensity from interfacial water OH band decreased steadily by increasing monovalent salt (NaCl, NaI) concentration due to counterion adsorption. On the other hand, ODA/water exhibited significantly smaller sum-frequency intensity than DPTAP/water, implying only small portion of protonated amine group (−NH3+) initially existed. By increasing the ionic strength, however, SF intensity of water OH band was enhanced markedly up to ∼1 mM, and then decreased in both NaCl and NaI solutions. By measuring the phase of the sum-frequency spectra, it was found that water dipoles under the ODA headgroup point downward, indicating that the surfaces were always positively charged. This demonstrated that increasing ionic strength facilitates protonation of primary amine headgroups. A simple model based on Poisson–Boltzmann (PB) theory explained this protonation behavior of primary amines.
中文翻译:

盐促进空气/水界面的胺基团的质子化
通过界面选择性振动和频生成光谱研究了由带电荷的Langmuir单层引起的界面水重定向,该Langmuir单层由伯脂肪胺(ODA)和具有季胺头基的阳离子脂质(DPTAP)组成。对于DPTAP单层,由于抗衡离子吸附,通过增加单价盐(NaCl,NaI)的浓度,最初来自界面水OH带的较大的总和频率强度逐渐降低。另一方面,ODA /水的总和频率强度明显小于DPTAP /水,这意味着质子化胺基团(-NH 3 +)最初存在。但是,通过增加离子强度,水OH谱带的SF强度显着增强,直至约1 mM,然后在NaCl和NaI溶液中均下降。通过测量和频谱的相位,发现ODA头基下方的水偶极子指向下方,表明表面始终带正电。这表明增加的离子强度有助于伯胺头基的质子化。一个基于泊松-玻耳兹曼(PB)理论的简单模型解释了伯胺的质子化行为。
更新日期:2017-07-22
中文翻译:

盐促进空气/水界面的胺基团的质子化
通过界面选择性振动和频生成光谱研究了由带电荷的Langmuir单层引起的界面水重定向,该Langmuir单层由伯脂肪胺(ODA)和具有季胺头基的阳离子脂质(DPTAP)组成。对于DPTAP单层,由于抗衡离子吸附,通过增加单价盐(NaCl,NaI)的浓度,最初来自界面水OH带的较大的总和频率强度逐渐降低。另一方面,ODA /水的总和频率强度明显小于DPTAP /水,这意味着质子化胺基团(-NH 3 +)最初存在。但是,通过增加离子强度,水OH谱带的SF强度显着增强,直至约1 mM,然后在NaCl和NaI溶液中均下降。通过测量和频谱的相位,发现ODA头基下方的水偶极子指向下方,表明表面始终带正电。这表明增加的离子强度有助于伯胺头基的质子化。一个基于泊松-玻耳兹曼(PB)理论的简单模型解释了伯胺的质子化行为。











































 京公网安备 11010802027423号
京公网安备 11010802027423号