当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Identification of a Robust Carbonyl Reductase for Diastereoselectively Building syn-3,5-Dihydroxy Hexanoate: a Bulky Side Chain of Atorvastatin
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2017-07-20 00:00:00 , DOI: 10.1021/acs.oprd.7b00194 Xu-Min Gong 1 , Gao-Wei Zheng 1 , You-Yan Liu 2, 3 , Jian-He Xu 1
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2017-07-20 00:00:00 , DOI: 10.1021/acs.oprd.7b00194 Xu-Min Gong 1 , Gao-Wei Zheng 1 , You-Yan Liu 2, 3 , Jian-He Xu 1
Affiliation
|
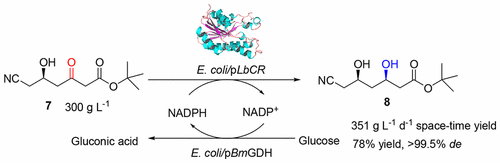
t-Butyl-6-cyano-(3R,5R)-dihydroxyhexanoate is an advanced chiral precursor for the synthesis of the side chain pharmacophore of cholesterol-lowering drug atorvastatin. Herein, a robust carbonyl reductase (LbCR) was newly identified from Lactobacillus brevis, which displays high activity and excellent diastereoselectivity toward bulky t-butyl 6-cyano-(5R)-hydroxy-3-oxo-hexanoate (7). The engineered Escherichia coli cells harboring LbCR and glucose dehydrogenase (for cofactor regeneration) were employed as biocatalysts for the asymmetric reduction of substrate 7. As a result, as much as 300 g L–1 of water-insoluble substrate was completely converted to the corresponding chiral diol with >99.5% de in a space–time yield of 351 g L–1 d–1, indicating a great potential of LbCR for practical synthesis of the very bulky and bi-chiral 3,5-dihydroxy carboxylate side chain of best-selling statin drugs.
中文翻译:

用于非对映选择性构建辛-3,5-二羟基己酸酯的稳健羰基还原酶的鉴定:阿托伐他汀的大体积侧链
叔丁基-6-氰基-(3 R,5 R)-二羟基己酸酯是一种先进的手性前体,用于合成降胆固醇药物阿托伐他汀的侧链药效团。在本文中,从短乳杆菌中新鉴定出一种强健的羰基还原酶(Lb CR),它对高密度的叔丁基6-氰基-(5 R)-羟基-3-氧代己酸叔丁酯具有很高的活性和非对映选择性(7)。具有Lb CR和葡萄糖脱氢酶(用于辅因子再生)的工程化大肠杆菌细胞被用作生物催化剂,用于不对称还原底物7。结果,多达300 g L –1的水不溶性底物以351 g L –1 d –1的时空产率被完全转化为相应的手性二元醇,de > 99.5%de,表明了巨大的潜力的Lb的CR对非常笨重和实际合成双向的畅销他汀类药物-手性3,5-二羟基羧酸侧链。
更新日期:2017-07-21
中文翻译:

用于非对映选择性构建辛-3,5-二羟基己酸酯的稳健羰基还原酶的鉴定:阿托伐他汀的大体积侧链
叔丁基-6-氰基-(3 R,5 R)-二羟基己酸酯是一种先进的手性前体,用于合成降胆固醇药物阿托伐他汀的侧链药效团。在本文中,从短乳杆菌中新鉴定出一种强健的羰基还原酶(Lb CR),它对高密度的叔丁基6-氰基-(5 R)-羟基-3-氧代己酸叔丁酯具有很高的活性和非对映选择性(7)。具有Lb CR和葡萄糖脱氢酶(用于辅因子再生)的工程化大肠杆菌细胞被用作生物催化剂,用于不对称还原底物7。结果,多达300 g L –1的水不溶性底物以351 g L –1 d –1的时空产率被完全转化为相应的手性二元醇,de > 99.5%de,表明了巨大的潜力的Lb的CR对非常笨重和实际合成双向的畅销他汀类药物-手性3,5-二羟基羧酸侧链。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号