Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Interruption of Hydrogen Bonding Networks of Water in Carbon Nanotubes Due to Strong Hydration Shell Formation
Langmuir ( IF 3.7 ) Pub Date : 2017-07-19 00:00:00 , DOI: 10.1021/acs.langmuir.7b01712 Yoshifumi Oya 1 , Kenji Hata 2 , Tomonori Ohba 1
Langmuir ( IF 3.7 ) Pub Date : 2017-07-19 00:00:00 , DOI: 10.1021/acs.langmuir.7b01712 Yoshifumi Oya 1 , Kenji Hata 2 , Tomonori Ohba 1
Affiliation
|
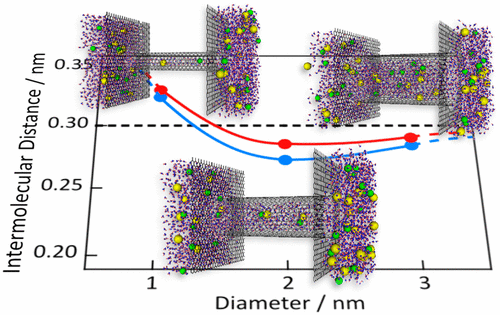
We present the structures of NaCl aqueous solution in carbon nanotubes with diameters of 1, 2, and 3 nm based on an analysis performed using X-ray diffraction and canonical ensemble Monte Carlo simulations. Anomalously longer nearest-neighbor distances were observed in the electrolyte for the 1-nm-diameter carbon nanotubes; in contrast, in the 2 and 3 nm carbon nanotubes, the nearest-neighbor distances were shorter than those in the bulk electrolyte. We also observed similar properties for water in carbon nanotubes, which was expected because the main component of the electrolyte was water. However, the nearest-neighbor distances of the electrolyte were longer than those of water in all of the carbon nanotubes; the difference was especially pronounced in the 2-nm-diameter carbon nanotubes. Thus, small numbers of ions affected the entire structure of the electrolyte in the nanopores of the carbon nanotubes. The formation of strong hydration shells between ions and water molecules considerably interrupted the hydrogen bonding between water molecules in the nanopores of the carbon nanotubes. The hydration shell had a diameter of approximately 1 nm, and hydration shells were thus adopted for the nanopores of the 2-nm-diameter carbon nanotubes, providing an explanation for the large difference in the nearest-neighbor distances between the electrolyte and water in these nanopores.
中文翻译:

由于强水化壳形成,碳纳米管中水的氢键网络中断。
基于使用X射线衍射和规范的整体Monte Carlo模拟进行的分析,我们介绍了直径分别为1、2和3 nm的碳纳米管中的NaCl水溶液的结构。对于直径为1 nm的碳纳米管,在电解液中观察到异常近邻距离更长。相反,在2nm和3nm的碳纳米管中,最接近的距离短于本体电解质中的距离。我们还观察到了碳纳米管中水的相似性质,这是可以预料的,因为电解质的主要成分是水。然而,在所有碳纳米管中,电解质的最接近距离比水更长。这种差异在直径为2 nm的碳纳米管中尤为明显。因此,少量离子会影响碳纳米管纳米孔中电解质的整个结构。离子与水分子之间形成坚固的水合壳,大大中断了碳纳米管纳米孔中水分子之间的氢键。水合壳的直径约为1 nm,因此水合壳被用于直径为2 nm的碳纳米管的纳米孔,从而解释了其中电解质和水之间的最接近距离之间的巨大差异纳米孔。
更新日期:2017-07-19
中文翻译:

由于强水化壳形成,碳纳米管中水的氢键网络中断。
基于使用X射线衍射和规范的整体Monte Carlo模拟进行的分析,我们介绍了直径分别为1、2和3 nm的碳纳米管中的NaCl水溶液的结构。对于直径为1 nm的碳纳米管,在电解液中观察到异常近邻距离更长。相反,在2nm和3nm的碳纳米管中,最接近的距离短于本体电解质中的距离。我们还观察到了碳纳米管中水的相似性质,这是可以预料的,因为电解质的主要成分是水。然而,在所有碳纳米管中,电解质的最接近距离比水更长。这种差异在直径为2 nm的碳纳米管中尤为明显。因此,少量离子会影响碳纳米管纳米孔中电解质的整个结构。离子与水分子之间形成坚固的水合壳,大大中断了碳纳米管纳米孔中水分子之间的氢键。水合壳的直径约为1 nm,因此水合壳被用于直径为2 nm的碳纳米管的纳米孔,从而解释了其中电解质和水之间的最接近距离之间的巨大差异纳米孔。































 京公网安备 11010802027423号
京公网安备 11010802027423号