Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Quinolinate Phosphoribosyltransferase is an Antiviral Host Factor Against Hepatitis C Virus Infection.
Scientific Reports ( IF 3.8 ) Pub Date : 2017-07-19 , DOI: 10.1038/s41598-017-06254-4 Zhilong Wang , Yanhang Gao , Chao Zhang , Haiming Hu , Dongwei Guo , Yi Xu , Qiuping Xu , Weihong Zhang , Sisi Deng , Pingyun Lv , Yan Yang , Yanhua Ding , Qingquan Li , Changjiang Weng , Xinwen Chen , Sitang Gong , Hairong Chen , Junqi Niu , Hong Tang
Scientific Reports ( IF 3.8 ) Pub Date : 2017-07-19 , DOI: 10.1038/s41598-017-06254-4 Zhilong Wang , Yanhang Gao , Chao Zhang , Haiming Hu , Dongwei Guo , Yi Xu , Qiuping Xu , Weihong Zhang , Sisi Deng , Pingyun Lv , Yan Yang , Yanhua Ding , Qingquan Li , Changjiang Weng , Xinwen Chen , Sitang Gong , Hairong Chen , Junqi Niu , Hong Tang
|
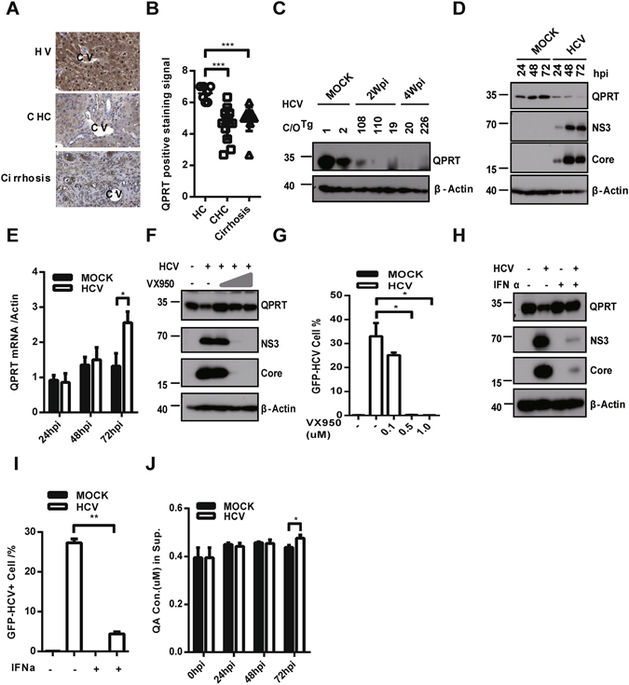
HCV infection can decrease NAD+/NADH ratio, which could convert lipid metabolism to favor HCV replication. In hepatocytes, quinolinate phosphoribosyl transferase (QPRT) catabolizes quinolinic acid (QA) to nicotinic acid mononucleotide (NAMN) for de novo NAD synthesis. However, whether and how HCV modulates QPRT hence the lipogenesis is unknown. In this work, we found QPRT was reduced significantly in livers of patients or humanized C/OTg mice with persistent HCV infection. Mechanistic studies indicated that HCV NS3/4A promoted proteasomal degradation of QPRT through Smurf2, an E3 ubiquitin-protein ligase, in Huh7.5.1 cells. Furthermore, QPRT enzymatic activity involved in suppression of HCV replication in cells. Activation of QPRT with clofibrate (CLO) or addition of QPRT catabolite NAD both inhibited HCV replication in cells, probably through NAD+-dependent Sirt1 inhibition of cellular lipogenesis. More importantly, administration of CLO, a hypolipidemic drug used in clinics, could significantly reduce the viral load in HCV infected C/OTg mice. Take together, these results suggested that HCV infection triggered proteasomal degradation of QPRT and consequently reduced de novo NAD synthesis and lipogenesis, in favor of HCV replication. Hepatic QPRT thus likely served as a cellular factor that dampened productive HCV replication.
中文翻译:

喹啉酸酯磷酸核糖基转移酶是抗丙型肝炎病毒感染的抗病毒宿主因子。
HCV感染可降低NAD + / NADH比率,这可将脂质代谢转化为有利于HCV复制的物质。在肝细胞中,喹啉酸磷酸核糖基转移酶(QPRT)将喹啉酸(QA)分解为烟酸单核苷酸(NAMN),从头合成NAD。然而,HCV是否以及如何调节QPRT,因此脂肪形成尚不清楚。在这项工作中,我们发现患者肝脏或人源化C / O Tg的QPRT显着降低HCV持续感染的小鼠。机理研究表明,HCV NS3 / 4A通过Huh7.5.1细胞中的E3泛素蛋白连接酶Smurf2促进了QPRT的蛋白酶体降解。此外,QPRT酶活性参与细胞中HCV复制的抑制。用氯贝特(CLO)激活QPRT或添加QPRT分解代谢产物NAD均可抑制细胞中HCV复制,可能是通过NAD +依赖性Sirt1抑制细胞脂肪生成。更重要的是,临床上使用的降血脂药物CLO的给药可以显着降低HCV感染的C / O Tg中的病毒载量老鼠。综上,这些结果表明,HCV感染触发了QPRT的蛋白酶体降解,并因此降低了从头NAD合成和脂肪生成的减少,有利于HCV复制。因此,肝脏QPRT可能是抑制生产性HCV复制的细胞因子。
更新日期:2017-07-20
中文翻译:

喹啉酸酯磷酸核糖基转移酶是抗丙型肝炎病毒感染的抗病毒宿主因子。
HCV感染可降低NAD + / NADH比率,这可将脂质代谢转化为有利于HCV复制的物质。在肝细胞中,喹啉酸磷酸核糖基转移酶(QPRT)将喹啉酸(QA)分解为烟酸单核苷酸(NAMN),从头合成NAD。然而,HCV是否以及如何调节QPRT,因此脂肪形成尚不清楚。在这项工作中,我们发现患者肝脏或人源化C / O Tg的QPRT显着降低HCV持续感染的小鼠。机理研究表明,HCV NS3 / 4A通过Huh7.5.1细胞中的E3泛素蛋白连接酶Smurf2促进了QPRT的蛋白酶体降解。此外,QPRT酶活性参与细胞中HCV复制的抑制。用氯贝特(CLO)激活QPRT或添加QPRT分解代谢产物NAD均可抑制细胞中HCV复制,可能是通过NAD +依赖性Sirt1抑制细胞脂肪生成。更重要的是,临床上使用的降血脂药物CLO的给药可以显着降低HCV感染的C / O Tg中的病毒载量老鼠。综上,这些结果表明,HCV感染触发了QPRT的蛋白酶体降解,并因此降低了从头NAD合成和脂肪生成的减少,有利于HCV复制。因此,肝脏QPRT可能是抑制生产性HCV复制的细胞因子。































 京公网安备 11010802027423号
京公网安备 11010802027423号