当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Formation of Highly Oxidized Radicals and Multifunctional Products from the Atmospheric Oxidation of Alkylbenzenes
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2017-07-18 00:00:00 , DOI: 10.1021/acs.est.7b02374
Sainan Wang 1, 2 , Runrun Wu 1 , Torsten Berndt 3 , Mikael Ehn 2 , Liming Wang 1, 4
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2017-07-18 00:00:00 , DOI: 10.1021/acs.est.7b02374
Sainan Wang 1, 2 , Runrun Wu 1 , Torsten Berndt 3 , Mikael Ehn 2 , Liming Wang 1, 4
Affiliation
|
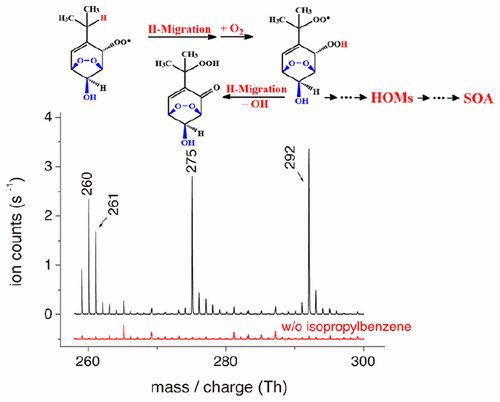
Aromatic hydrocarbons contribute significantly to tropospheric ozone and secondary organic aerosols (SOA). Despite large efforts in elucidating the formation mechanism of aromatic-derived SOA, current models still substantially underestimate the SOA yields when comparing to field measurements. Here we present a new, up to now undiscovered pathway for the formation of highly oxidized products from the OH-initiated oxidation of alkyl benzenes based on theoretical and experimental investigations. We propose that unimolecular H-migration followed by O2-addition, a so-called autoxidation step, can take place in bicyclic peroxy radicals (BPRs), which are important intermediates of the OH-initiated oxidation of aromatic compounds. These autoxidation steps lead to the formation of highly oxidized multifunctional compounds (HOMs), which are able to form SOA. Our theoretical calculations suggest that the intramolecular H-migration in BPRs of substituted benzenes could be fast enough to compete with bimolecular reactions with HO2 radicals or NO under atmospheric conditions. The theoretical findings are experimentally supported by flow tube studies using chemical ionization mass spectrometry to detect the highly oxidized peroxy radical intermediates and closed-shell products. This new unimolecular BPR route to form HOMs in the gas phase enhances our understanding of the aromatic oxidation mechanism, and contributes significantly to a better understanding of aromatic-derived SOA in urban areas.
中文翻译:

烷基苯的大气氧化形成高氧化的自由基和多功能产物
芳香烃对对流层臭氧和二次有机气溶胶(SOA)的贡献很大。尽管在阐明芳族衍生SOA的形成机理方面进行了大量努力,但与现场测量相比,当前模型仍大大低估了SOA产量。在这里,我们根据理论和实验研究,提出了一种由OH引发的烷基苯氧化生成高度氧化产物的新途径,至今尚未发现。我们建议单分子H迁移后跟O 2-加成反应,即所谓的自氧化步骤,可以在双环过氧自由基(BPR)中发生,其为OH-引发的芳族化合物氧化的重要中间体。这些自氧化步骤导致形成能够形成SOA的高度氧化的多功能化合物(HOM)。我们的理论计算表明,取代苯的BPR中的分子内H迁移可能足以与HO 2的双分子反应竞争。自由基或一氧化氮在大气条件下。理论发现得到了使用化学电离质谱法检测高氧化过氧自由基中间体和闭壳产物的流管研究的实验支持。这种在气相中形成HOM的新的单分子BPR途径增强了我们对芳族氧化机理的理解,并极大地有助于更好地理解城市地区的芳族衍生SOA。
更新日期:2017-07-19
中文翻译:

烷基苯的大气氧化形成高氧化的自由基和多功能产物
芳香烃对对流层臭氧和二次有机气溶胶(SOA)的贡献很大。尽管在阐明芳族衍生SOA的形成机理方面进行了大量努力,但与现场测量相比,当前模型仍大大低估了SOA产量。在这里,我们根据理论和实验研究,提出了一种由OH引发的烷基苯氧化生成高度氧化产物的新途径,至今尚未发现。我们建议单分子H迁移后跟O 2-加成反应,即所谓的自氧化步骤,可以在双环过氧自由基(BPR)中发生,其为OH-引发的芳族化合物氧化的重要中间体。这些自氧化步骤导致形成能够形成SOA的高度氧化的多功能化合物(HOM)。我们的理论计算表明,取代苯的BPR中的分子内H迁移可能足以与HO 2的双分子反应竞争。自由基或一氧化氮在大气条件下。理论发现得到了使用化学电离质谱法检测高氧化过氧自由基中间体和闭壳产物的流管研究的实验支持。这种在气相中形成HOM的新的单分子BPR途径增强了我们对芳族氧化机理的理解,并极大地有助于更好地理解城市地区的芳族衍生SOA。

































 京公网安备 11010802027423号
京公网安备 11010802027423号