当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structure of Human Tyrosinase Related Protein 1 Reveals a Binuclear Zinc Active Site Important for Melanogenesis
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2017-07-17 06:55:49 , DOI: 10.1002/anie.201704616 Xuelei Lai 1, 2 , Harry J. Wichers 3 , Montserrat Soler‐Lopez 2 , Bauke W. Dijkstra 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2017-07-17 06:55:49 , DOI: 10.1002/anie.201704616 Xuelei Lai 1, 2 , Harry J. Wichers 3 , Montserrat Soler‐Lopez 2 , Bauke W. Dijkstra 1
Affiliation
|
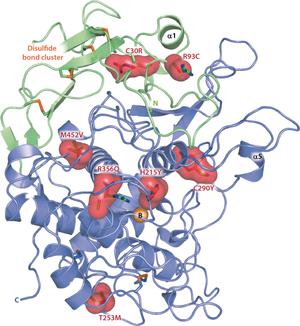
Tyrosinase-related protein 1 (TYRP1) is one of three tyrosinase-like glycoenzymes in human melanocytes that are key to the production of melanin, the compound responsible for the pigmentation of skin, eye, and hair. Difficulties with producing these enzymes in pure form have hampered the understanding of their activity and the effect of mutations that cause albinism and pigmentation disorders. Herein we show that the typical tyrosinase-like subdomain of TYRP1 contains two zinc ions in the active site instead of copper ions as found in tyrosinases, which explains why TYRP1 does not exhibit tyrosinase redox activity. In addition, the structures reveal for the first time that the Cys-rich subdomain, which is unique to vertebrate melanogenic proteins, has an epidermal growth factor-like fold and is tightly associated with the tyrosinase subdomain. Our structures suggest that most albinism-related mutations of TYRP1 affect its stability or activity.
中文翻译:

人类酪氨酸酶相关蛋白1的结构揭示了重要的黑色素生成的双核锌活性位点。
酪氨酸酶相关蛋白1(TYRP1)是人黑素细胞中三种类似于酪氨酸酶的糖酶之一,是产生黑色素的关键,黑色素是负责皮肤,眼睛和头发色素沉着的化合物。以纯净形式生产这些酶的困难已经阻碍了人们对其活性以及引起白化病和色素沉着症的突变的影响的理解。本文中,我们显示了TYRP1的典型酪氨酸酶样亚结构域在活性位点包含两个锌离子,而不是酪氨酸酶中发现的铜离子,这解释了TYRP1不显示酪氨酸酶氧化还原活性的原因。另外,该结构首次揭示了富含脊椎动物的黑色素生成蛋白所特有的富含Cys的亚结构域,具有表皮生长因子样的折叠,并与酪氨酸酶亚结构域紧密相关。
更新日期:2017-07-17
中文翻译:

人类酪氨酸酶相关蛋白1的结构揭示了重要的黑色素生成的双核锌活性位点。
酪氨酸酶相关蛋白1(TYRP1)是人黑素细胞中三种类似于酪氨酸酶的糖酶之一,是产生黑色素的关键,黑色素是负责皮肤,眼睛和头发色素沉着的化合物。以纯净形式生产这些酶的困难已经阻碍了人们对其活性以及引起白化病和色素沉着症的突变的影响的理解。本文中,我们显示了TYRP1的典型酪氨酸酶样亚结构域在活性位点包含两个锌离子,而不是酪氨酸酶中发现的铜离子,这解释了TYRP1不显示酪氨酸酶氧化还原活性的原因。另外,该结构首次揭示了富含脊椎动物的黑色素生成蛋白所特有的富含Cys的亚结构域,具有表皮生长因子样的折叠,并与酪氨酸酶亚结构域紧密相关。






























 京公网安备 11010802027423号
京公网安备 11010802027423号