当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Accelerated Reduction of Bromate in Frozen Solution
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2017-07-13 00:00:00 , DOI: 10.1021/acs.est.7b00915
Dae Wi Min 1 , Wonyong Choi 1
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2017-07-13 00:00:00 , DOI: 10.1021/acs.est.7b00915
Dae Wi Min 1 , Wonyong Choi 1
Affiliation
|
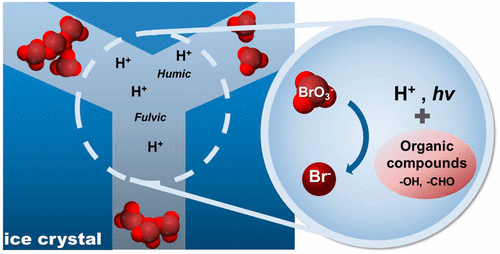
Bromate is a common disinfection byproduct formed during ozonation. Reducing bromate into bromide can remove this toxic pollutant, however, not many studies have been done for its environmental fate. In this work, we demonstrate a new transformation pathway that bromate can be efficiently reduced to bromide in frozen solution in the presence of organic reductants like humic substances (HS). The results showed that bromate in frozen solution could be removed by 30–40% in dark condition and 80–90% in irradiation condition (λ > 300 nm) in 24 h, while around 1% bromate was reduced in aqueous solution. The bromate reduction by HS induced a partial oxidation of HS, which was confirmed by X-ray photoelectron spectroscopic analysis of the HS sample recovered from the frozen solution. Photoluminescence analysis of HS revealed that the fluorescence quenching by bromate was observed only with very high concentration of bromate (0.1–0.2 M) in aqueous solution whereas the quenching effect in frozen solution was seen with much lower bromate concentration (5–100 μM). The highly enhanced removal of bromate in ice is ascribed to the freeze concentration effect that bromate and HS are concentrated by orders of magnitude to accelerate the bimolecular transformation in the ice grain boundary region. Freezing process in cold environments would provide a unique chemical mechanism for the removal of persistent bromate.
中文翻译:

加速还原冷冻溶液中的溴酸盐
溴酸盐是在臭氧化过程中形成的常见消毒副产物。将溴酸盐还原为溴化物可以除去这种有毒污染物,但是,关于其环境命运的研究还很少。在这项工作中,我们证明了一种新的转化途径,即在有机还原剂(如腐殖质(HS))存在下,溴酸盐可以在冷冻溶液中有效还原为溴化物。结果表明,在黑暗条件下,冷冻溶液中的溴酸盐在24 h内可去除30–40%,在辐照条件下(λ> 300 nm)可去除80–90%,而水溶液中的溴酸盐减少约1%。HS对溴酸盐的还原引起了HS的部分氧化,这已通过X射线光电子能谱分析从冷冻溶液中回收的HS样品得到了证实。HS的光致发光分析表明,仅在水溶液中溴酸盐浓度很高(0.1–0.2 M)时,才观察到溴酸盐的荧光猝灭,而在溴酸盐浓度低得多(5–100μM)时,观察到冷冻溶液中的猝灭作用。冰中溴酸盐的去除率大大提高归因于冻结浓缩效应,即溴酸盐和HS浓缩了几个数量级,从而加速了冰晶边界区域中的双分子转化。寒冷环境中的冷冻过程将为去除持久性溴酸盐提供独特的化学机理。冰中溴酸盐的去除率大大提高归因于冻结浓缩效应,即溴酸盐和HS浓缩了几个数量级,从而加速了冰晶边界区域中的双分子转化。寒冷环境中的冷冻过程将为去除持久性溴酸盐提供独特的化学机理。冰中溴酸盐的去除率大大提高归因于冻结浓缩效应,即溴酸盐和HS浓缩了几个数量级,从而加速了冰晶边界区域中的双分子转化。寒冷环境中的冷冻过程将为去除持久性溴酸盐提供独特的化学机理。
更新日期:2017-07-14
中文翻译:

加速还原冷冻溶液中的溴酸盐
溴酸盐是在臭氧化过程中形成的常见消毒副产物。将溴酸盐还原为溴化物可以除去这种有毒污染物,但是,关于其环境命运的研究还很少。在这项工作中,我们证明了一种新的转化途径,即在有机还原剂(如腐殖质(HS))存在下,溴酸盐可以在冷冻溶液中有效还原为溴化物。结果表明,在黑暗条件下,冷冻溶液中的溴酸盐在24 h内可去除30–40%,在辐照条件下(λ> 300 nm)可去除80–90%,而水溶液中的溴酸盐减少约1%。HS对溴酸盐的还原引起了HS的部分氧化,这已通过X射线光电子能谱分析从冷冻溶液中回收的HS样品得到了证实。HS的光致发光分析表明,仅在水溶液中溴酸盐浓度很高(0.1–0.2 M)时,才观察到溴酸盐的荧光猝灭,而在溴酸盐浓度低得多(5–100μM)时,观察到冷冻溶液中的猝灭作用。冰中溴酸盐的去除率大大提高归因于冻结浓缩效应,即溴酸盐和HS浓缩了几个数量级,从而加速了冰晶边界区域中的双分子转化。寒冷环境中的冷冻过程将为去除持久性溴酸盐提供独特的化学机理。冰中溴酸盐的去除率大大提高归因于冻结浓缩效应,即溴酸盐和HS浓缩了几个数量级,从而加速了冰晶边界区域中的双分子转化。寒冷环境中的冷冻过程将为去除持久性溴酸盐提供独特的化学机理。冰中溴酸盐的去除率大大提高归因于冻结浓缩效应,即溴酸盐和HS浓缩了几个数量级,从而加速了冰晶边界区域中的双分子转化。寒冷环境中的冷冻过程将为去除持久性溴酸盐提供独特的化学机理。

































 京公网安备 11010802027423号
京公网安备 11010802027423号