当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Phosphonium Salts as Pseudohalides: Regioselective Nickel-Catalyzed Cross-Coupling of Complex Pyridines and Diazines
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2017-07-13 07:56:33 , DOI: 10.1002/anie.201704948 Xuan Zhang 1 , Andrew McNally 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2017-07-13 07:56:33 , DOI: 10.1002/anie.201704948 Xuan Zhang 1 , Andrew McNally 1
Affiliation
|
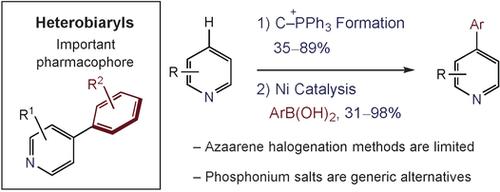
Heterobiaryls are important pharmacophores that are challenging to prepare by traditional cross-coupling methods. An alternative approach is presented where pyridines and diazines are converted into heteroaryl phosphonium salts and coupled with aryl boronic acids. Nickel catalysts are unique for selective heteroaryl transfer, and the reaction has a broad substrate scope that includes complex pharmaceuticals. Phosphonium ions also display orthogonal reactivity in cross-couplings compared to halides, enabling chemoselective palladium- and nickel-catalyzed coupling sequences.
中文翻译:

磷盐作为伪卤化物:复杂吡啶和二嗪的区域选择性镍催化交叉偶联。
杂二芳基化合物是重要的药效团,通过传统的交叉偶联方法难以制备。提出了一种替代方法,其中将吡啶和二嗪转化为杂芳基phospho盐并与芳基硼酸偶联。镍催化剂对于选择性杂芳基转移是独特的,该反应具有广泛的底物范围,包括复杂的药物。与卤化物相比,离子在交叉偶联中也表现出正交反应性,从而实现化学选择性的钯和镍催化的偶联序列。
更新日期:2017-07-13
中文翻译:

磷盐作为伪卤化物:复杂吡啶和二嗪的区域选择性镍催化交叉偶联。
杂二芳基化合物是重要的药效团,通过传统的交叉偶联方法难以制备。提出了一种替代方法,其中将吡啶和二嗪转化为杂芳基phospho盐并与芳基硼酸偶联。镍催化剂对于选择性杂芳基转移是独特的,该反应具有广泛的底物范围,包括复杂的药物。与卤化物相比,离子在交叉偶联中也表现出正交反应性,从而实现化学选择性的钯和镍催化的偶联序列。






























 京公网安备 11010802027423号
京公网安备 11010802027423号