当前位置:
X-MOL 学术
›
Energy Environ. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Intercalation of solvated Na-ions into graphite
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2017-05-31 00:00:00 , DOI: 10.1039/c7ee00546f L. Seidl 1, 2, 3, 4, 5 , N. Bucher 6, 7, 8 , E. Chu 2, 3, 4, 9 , S. Hartung 6, 7, 8 , S. Martens 2, 3, 4, 5, 9 , O. Schneider 2, 3, 4, 5, 9 , U. Stimming 1, 2, 3, 4, 5
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2017-05-31 00:00:00 , DOI: 10.1039/c7ee00546f L. Seidl 1, 2, 3, 4, 5 , N. Bucher 6, 7, 8 , E. Chu 2, 3, 4, 9 , S. Hartung 6, 7, 8 , S. Martens 2, 3, 4, 5, 9 , O. Schneider 2, 3, 4, 5, 9 , U. Stimming 1, 2, 3, 4, 5
Affiliation
|
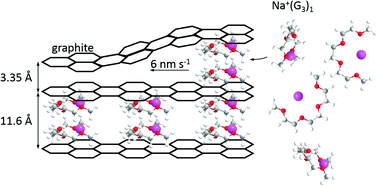
The reversible intercalation of solvated Na-ions into graphite and the concomitant formation of ternary Na–graphite intercalation compounds (GICs) are studied using several in operando techniques, such as X-ray-diffraction (XRD), electrochemical scanning tunnelling microscopy (EC-STM) and electrochemical quartz crystal microbalance techniques (EQCM). Linear ethylene glycol dimethyl ether homologues (“glymes”) Gx with x + 1 O-atoms were used as solvents, where x is 1–4. The intercalation mechanism of Na+(Gx)y-complexes was investigated with a focus on the phase transitions and diffusion rates of the Na+(Gx)y-complexes inside the graphite lattice. For the four shortest glymes (G1 to G4), it is found using XRD that an intermediate stage 2 Na–GIC (NaC48) is formed upon the partial sodiation of the graphite electrode. At full sodiation stage 1 Na–GIC (NaC18, 112 mA h g−1) is obtained for G1, G2 and G4, while the G3 system also forms a stage 1 Na–GIC but with less Na incorporated (NaC30, 70 mA h g−1). The phase transitions of a battery electrode upon ion-intercalation are visualised using STM on the atomic scale for the first time. In addition, the local diffusion rates of the intercalated species inside the electrode were determined, a unique approach for determining kinetic effects in batteries on the atomic scale. The formation of a solid electrolyte interphase (SEI) is observed with STM as well as with an EQCM, while the latter technique is used for novel in situ hydrodynamic spectroscopy, giving further insight into the intercalation mechanism.
中文翻译:

溶剂化的钠离子嵌入石墨中
使用几种操作技术,例如X射线衍射(XRD),电化学扫描隧道显微镜(EC- STM)和电化学石英晶体微量天平技术(EQCM)。使用具有x + 1个O原子的线性乙二醇二甲醚同系物(“甘醇二甲酸酯”)G x作为溶剂,其中x为1-4。研究了Na +(G x)y络合物的嵌入机理,重点研究了Na +(G x)的相变和扩散速率。y-石墨晶格内部的络合物。对于四种最短的甘醇二甲醚(G 1至G 4),使用XRD发现,在石墨电极的部分糊化过程中形成了中间级2 Na–GIC(NaC 48)。在全sodiation阶段1的Na-GIC(NAC 18,112毫安汞柱-1)是针对G得到1,G 2和G 4,而G 3系统还形成阶段1的Na-GIC但较少的Na并入(NAC 30,70毫安汞柱-1)。首次使用原子尺度上的STM可视化离子插入时电池电极的相变。此外,还确定了电极内部插层物质的局部扩散速率,这是一种用于确定电池中原子级动力学效应的独特方法。用STM以及EQCM观察到了固体电解质中间相(SEI)的形成,而后一种技术被用于新颖的原位流体动力学光谱分析,从而进一步了解了插入机理。
更新日期:2017-07-13
中文翻译:

溶剂化的钠离子嵌入石墨中
使用几种操作技术,例如X射线衍射(XRD),电化学扫描隧道显微镜(EC- STM)和电化学石英晶体微量天平技术(EQCM)。使用具有x + 1个O原子的线性乙二醇二甲醚同系物(“甘醇二甲酸酯”)G x作为溶剂,其中x为1-4。研究了Na +(G x)y络合物的嵌入机理,重点研究了Na +(G x)的相变和扩散速率。y-石墨晶格内部的络合物。对于四种最短的甘醇二甲醚(G 1至G 4),使用XRD发现,在石墨电极的部分糊化过程中形成了中间级2 Na–GIC(NaC 48)。在全sodiation阶段1的Na-GIC(NAC 18,112毫安汞柱-1)是针对G得到1,G 2和G 4,而G 3系统还形成阶段1的Na-GIC但较少的Na并入(NAC 30,70毫安汞柱-1)。首次使用原子尺度上的STM可视化离子插入时电池电极的相变。此外,还确定了电极内部插层物质的局部扩散速率,这是一种用于确定电池中原子级动力学效应的独特方法。用STM以及EQCM观察到了固体电解质中间相(SEI)的形成,而后一种技术被用于新颖的原位流体动力学光谱分析,从而进一步了解了插入机理。






























 京公网安备 11010802027423号
京公网安备 11010802027423号