当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
PEGylated, Water-Soluble, Stable Aminyl Radical
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2017-07-12 00:00:00 , DOI: 10.1021/acs.joc.7b01212
Ying Wang 1 , Suchada Rajca 1 , Andrzej Rajca 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2017-07-12 00:00:00 , DOI: 10.1021/acs.joc.7b01212
Ying Wang 1 , Suchada Rajca 1 , Andrzej Rajca 1
Affiliation
|
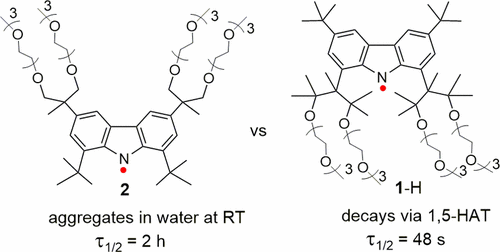
We report the synthesis and kinetic study of PEGylated, water-soluble aminyl radical 2. The radical possesses four mPEG-3 groups replacing four methyl groups in the tert-butyl groups at the 3- and 6-positions of 1,3,6,8-tetra-tert-butyl carbazyl (TTBC). This structure is designed to mitigate the rapid decomposition of the radical via intramolecular 1,5-hydrogen atom transfer (1,5-HAT) that was observed in its constitutional isomer 1-H with four mPEG-3 groups in the vicinity of the nitrogen-centered radical (1- and 8-positions of TTBC). In dry, degassed acetone at 295 K, the radical 2 has a half-life, τ1/2 = 49 h (ΔH‡ = 17.9 ± 0.8 kcal mol–1), which is 3 orders of magnitude longer than that for 1-H, which decays via 1,5-HAT (τ1/2 = 48 s, ΔH‡ = 10.0 ± 0.3 kcal mol–1). Aminyl radical 2 aggregates at ambient conditions in water and has a half-life, τ1/2 = 2 h.
中文翻译:

聚乙二醇化,水溶性,稳定的氨基自由基
我们报告了聚乙二醇化的水溶性氨基自由基2的合成和动力学研究。自由基具有4 MPEG-3基团取代在四个甲基叔丁基基团的1,3,6,8-四的3-和6-位叔丁基咔唑(TTBC)。设计该结构的目的是减轻自由基通过分子内1,5-氢原子转移(1,5-HAT)的快速分解,这是在其结构异构体1 -H中在氮附近带有四个mPEG-3基团观察到的中心自由基(TTBC的1和8位)。在295 K的干燥,脱气的丙酮中,自由基2的半衰期为τ1 /2 = 49 h(ΔH ‡ = 17.9±0.8 kcal mol–1),比1 -H长3个数量级,后者通过1,5-HAT衰减(τ1 /2 = 48 s,ΔH ‡ = 10.0±0.3 kcal mol –1)。氨基自由基2在环境条件下在水中聚集,半衰期为τ1 /2 = 2 h。
更新日期:2017-07-13
中文翻译:

聚乙二醇化,水溶性,稳定的氨基自由基
我们报告了聚乙二醇化的水溶性氨基自由基2的合成和动力学研究。自由基具有4 MPEG-3基团取代在四个甲基叔丁基基团的1,3,6,8-四的3-和6-位叔丁基咔唑(TTBC)。设计该结构的目的是减轻自由基通过分子内1,5-氢原子转移(1,5-HAT)的快速分解,这是在其结构异构体1 -H中在氮附近带有四个mPEG-3基团观察到的中心自由基(TTBC的1和8位)。在295 K的干燥,脱气的丙酮中,自由基2的半衰期为τ1 /2 = 49 h(ΔH ‡ = 17.9±0.8 kcal mol–1),比1 -H长3个数量级,后者通过1,5-HAT衰减(τ1 /2 = 48 s,ΔH ‡ = 10.0±0.3 kcal mol –1)。氨基自由基2在环境条件下在水中聚集,半衰期为τ1 /2 = 2 h。

































 京公网安备 11010802027423号
京公网安备 11010802027423号