当前位置:
X-MOL 学术
›
J. Proteome Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A PLC-γ1 Feedback Pathway Regulates Lck Substrate Phosphorylation at the T-Cell Receptor and SLP-76 Complex
Journal of Proteome Research ( IF 3.8 ) Pub Date : 2017-07-06 00:00:00 , DOI: 10.1021/acs.jproteome.6b01026 Judson Belmont 1 , Tao Gu 1 , Ashley Mudd 1 , Arthur R. Salomon 1, 2
Journal of Proteome Research ( IF 3.8 ) Pub Date : 2017-07-06 00:00:00 , DOI: 10.1021/acs.jproteome.6b01026 Judson Belmont 1 , Tao Gu 1 , Ashley Mudd 1 , Arthur R. Salomon 1, 2
Affiliation
|
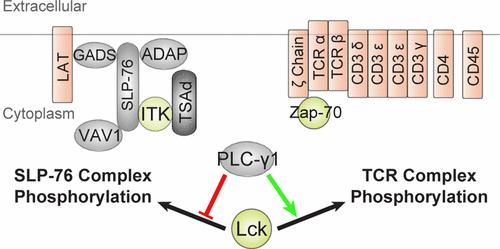
Phospholipase C gamma 1 (PLC-γ1) occupies a critically important position in the T-cell signaling pathway. While its functions as a regulator of both Ca2+ signaling and PKC-family kinases are well characterized, PLC-γ1’s role in the regulation of early T-cell receptor signaling events is incompletely understood. Activation of the T-cell receptor leads to the formation of a signalosome complex between SLP-76, LAT, PLC-γ1, Itk, and Vav1. Recent studies have revealed the existence of both positive and negative feedback pathways from SLP-76 to the apical kinase in the pathway, Lck. To determine if PLC-γ1 contributes to the regulation of these feedback networks, we performed a quantitative phosphoproteomic analysis of PLC-γ1-deficient T cells. These data revealed a previously unappreciated role for PLC-γ1 in the positive regulation of Zap-70 and T-cell receptor tyrosine phosphorylation. Conversely, PLC-γ1 negatively regulated the phosphorylation of SLP-76-associated proteins, including previously established Lck substrate phosphorylation sites within this complex. While the positive and negative regulatory phosphorylation sites on Lck were largely unchanged, Tyr192 phosphorylation was elevated in Jgamma1. The data supports a model wherein Lck’s targeting, but not its kinase activity, is altered by PLC-γ1, possibly through Lck Tyr192 phosphorylation and increased association of the kinase with protein scaffolds SLP-76 and TSAd.
中文翻译:

PLC-γ1反馈途径调节T细胞受体和SLP-76复合物上Lck底物的磷酸化
磷脂酶Cγ1(PLC-γ1)在T细胞信号传导途径中占有至关重要的位置。虽然它同时充当Ca 2+和Ca 2+的调节剂信号转导和PKC家族激酶已被很好地表征,PLC-γ1在早期T细胞受体信号转导事件调控中的作用尚不完全清楚。T细胞受体的激活导致SLP-76,LAT,PLC-γ1,Itk和Vav1之间形成信号体复合物。最近的研究揭示了从SLP-76到Lck途径中顶激酶的正反馈和负反馈途径均存在。为了确定PLC-γ1是否有助于调节这些反馈网络,我们对PLC-γ1缺陷型T细胞进行了磷酸化蛋白质组学定量分析。这些数据揭示了PLC-γ1在Zap-70和T细胞受体酪氨酸磷酸化正调控中的前所未有的作用。相反,PLC-γ1负调控SLP-76相关蛋白的磷酸化,包括先前在该复合物中建立的Lck底物磷酸化位点。虽然Lck上的正负调节磷酸化位点基本未变,但Tyr在Jgamma1中192磷酸化升高。数据支持了一个模型,其中PLC-γ1可能通过Lck Tyr 192磷酸化以及激酶与蛋白支架SLP-76和TSAd的缔合增加来改变Lck的靶向作用,但不改变其激酶活性。
更新日期:2017-07-12
中文翻译:

PLC-γ1反馈途径调节T细胞受体和SLP-76复合物上Lck底物的磷酸化
磷脂酶Cγ1(PLC-γ1)在T细胞信号传导途径中占有至关重要的位置。虽然它同时充当Ca 2+和Ca 2+的调节剂信号转导和PKC家族激酶已被很好地表征,PLC-γ1在早期T细胞受体信号转导事件调控中的作用尚不完全清楚。T细胞受体的激活导致SLP-76,LAT,PLC-γ1,Itk和Vav1之间形成信号体复合物。最近的研究揭示了从SLP-76到Lck途径中顶激酶的正反馈和负反馈途径均存在。为了确定PLC-γ1是否有助于调节这些反馈网络,我们对PLC-γ1缺陷型T细胞进行了磷酸化蛋白质组学定量分析。这些数据揭示了PLC-γ1在Zap-70和T细胞受体酪氨酸磷酸化正调控中的前所未有的作用。相反,PLC-γ1负调控SLP-76相关蛋白的磷酸化,包括先前在该复合物中建立的Lck底物磷酸化位点。虽然Lck上的正负调节磷酸化位点基本未变,但Tyr在Jgamma1中192磷酸化升高。数据支持了一个模型,其中PLC-γ1可能通过Lck Tyr 192磷酸化以及激酶与蛋白支架SLP-76和TSAd的缔合增加来改变Lck的靶向作用,但不改变其激酶活性。















































 京公网安备 11010802027423号
京公网安备 11010802027423号