当前位置:
X-MOL 学术
›
J. Chem. Educ.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cross-Course Collaboration in the Undergraduate Chemistry Curriculum: Primary Kinetic Isotope Effect in the Hypochlorite Oxidation of 1-Phenylethanol in the Physical Chemistry Laboratory
Journal of Chemical Education ( IF 2.5 ) Pub Date : 2017-06-23 00:00:00 , DOI: 10.1021/acs.jchemed.6b00950
Robert J. Noll 1 , Richard W. Fitch 1 , Richard A. Kjonaas 1 , Richard A. Wyatt 1
Journal of Chemical Education ( IF 2.5 ) Pub Date : 2017-06-23 00:00:00 , DOI: 10.1021/acs.jchemed.6b00950
Robert J. Noll 1 , Richard W. Fitch 1 , Richard A. Kjonaas 1 , Richard A. Wyatt 1
Affiliation
|
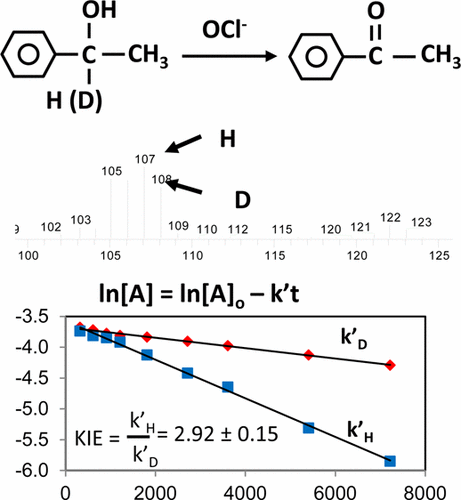
A kinetic isotope effect (KIE) experiment is described for the physical chemistry laboratory. Students conduct a hypochlorite (household bleach) oxidation of an equimolar mixture of 1-phenylethanol and 1-deuterio-1-phenylethanol to acetophenone. The reaction occurs in a biphasic reaction mixture and follows first-order kinetics with respect to either isotopomer of 1-phenylethanol. Reaction progress is measured by gas chromatography–mass spectrometry (GC–MS). Alternatively, the experiment could be conducted with each isotopomer serially and followed by GC alone. The reaction rate constant for the disappearance of 1-phenylethanol, kH, ranges from 3 × 10–4 to 2 × 10–3 s–1, while kD, for 1-deuterio-1-phenylethanol, ranges from 9 × 10–5 to 5 × 10–4 s–1. The observed KIE, the ratio kH/kD, is remarkably robust, ranging between 2.3 and 3.6, with a mean of 2.9 and standard deviation of 0.4 over three years of student data. The robustness of the observed KIE stems from using competing reactions. The experiment can be completed in about 3 h; GC–MS data is conveniently acquired overnight using an autosampler. The experiment, as presented here, can stand alone, but is well-suited to cross-course collaboration between the organic and physical chemistry laboratories. The preceding companion paper describes the synthesis of 1-phenylethanol and 1-deuterio-1-phenylethanol using borohydride or borodeuteride reduction of acetophenone as an experiment for the organic laboratory.
中文翻译:

本科化学课程中的跨课程合作:物理化学实验室中1-苯基乙醇的次氯酸盐氧化中的主要动力学同位素效应
物理化学实验室描述了动力学同位素效应(KIE)实验。学生将1-苯基乙醇和1-氘-1-苯基乙醇的等摩尔混合物的次氯酸盐(家用漂白剂)氧化为苯乙酮。该反应在两相反应混合物中进行,并且相对于1-苯基乙醇的任一异构体遵循一级动力学。反应进程通过气相色谱-质谱法(GC-MS)进行测量。或者,可以依次对每种同功异构体进行实验,然后单独进行GC。1-苯基乙醇消失的反应速率常数k H在3×10 –4至2×10 –3 s –1范围内,而k D对于1-氘-1-苯基乙醇,范围为9×10 –5到5×10 –4 s –1。观测到的KIE,比率k H / k D,在三年的学生数据中表现出色,介于2.3到3.6之间,平均值为2.9,标准差为0.4。观察到的KIE的稳健性源于使用竞争性反应。实验可以在大约3小时内完成;使用自动进样器可在一夜之间方便地获取GC-MS数据。如此处所示,该实验可以独立进行,但非常适合有机化学实验室和物理化学实验室之间的跨学科协作。先前的随附论文描述了使用苯乙酮的硼氢化物或硼氘化物还原法作为有机实验室的实验来合成1-苯基乙醇和1-氘代-1-苯基乙醇。
更新日期:2017-06-23
中文翻译:

本科化学课程中的跨课程合作:物理化学实验室中1-苯基乙醇的次氯酸盐氧化中的主要动力学同位素效应
物理化学实验室描述了动力学同位素效应(KIE)实验。学生将1-苯基乙醇和1-氘-1-苯基乙醇的等摩尔混合物的次氯酸盐(家用漂白剂)氧化为苯乙酮。该反应在两相反应混合物中进行,并且相对于1-苯基乙醇的任一异构体遵循一级动力学。反应进程通过气相色谱-质谱法(GC-MS)进行测量。或者,可以依次对每种同功异构体进行实验,然后单独进行GC。1-苯基乙醇消失的反应速率常数k H在3×10 –4至2×10 –3 s –1范围内,而k D对于1-氘-1-苯基乙醇,范围为9×10 –5到5×10 –4 s –1。观测到的KIE,比率k H / k D,在三年的学生数据中表现出色,介于2.3到3.6之间,平均值为2.9,标准差为0.4。观察到的KIE的稳健性源于使用竞争性反应。实验可以在大约3小时内完成;使用自动进样器可在一夜之间方便地获取GC-MS数据。如此处所示,该实验可以独立进行,但非常适合有机化学实验室和物理化学实验室之间的跨学科协作。先前的随附论文描述了使用苯乙酮的硼氢化物或硼氘化物还原法作为有机实验室的实验来合成1-苯基乙醇和1-氘代-1-苯基乙醇。































 京公网安备 11010802027423号
京公网安备 11010802027423号