当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Phase Diagrams and Physicochemical Properties for the Ternary System (CsCl + NaCl + H2O) at T = (298.15, 308.15, and 318.15) K
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2017-03-27 00:00:00 , DOI: 10.1021/acs.jced.7b00023 Yunxue Gao 1 , Shuni Li 1 , Quanguo Zhai 1 , Yucheng Jiang 1 , Mancheng Hu 1
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2017-03-27 00:00:00 , DOI: 10.1021/acs.jced.7b00023 Yunxue Gao 1 , Shuni Li 1 , Quanguo Zhai 1 , Yucheng Jiang 1 , Mancheng Hu 1
Affiliation
|
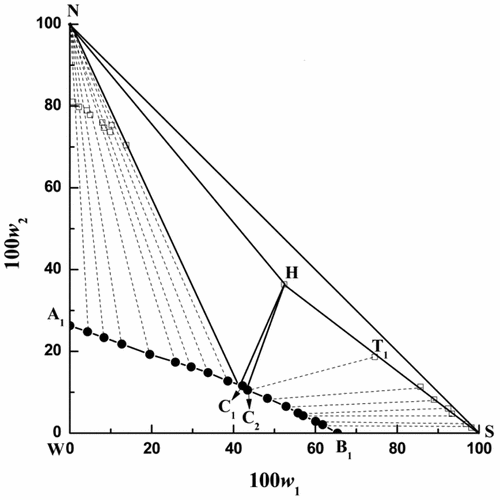
Complete phase diagrams and physico-chemical properties for the ternary system (CsCl + NaCl + H2O) were determined at T = (298.15, 308.15, and 318.15) K, respectively. The X-ray diffraction method and Schreinemaker’s wet residue method were used to verify the solid phase. The phase diagrams show this ternary system is a complex type. Three crystallization regions were found corresponding to the single salt NaCl, a compound CsCl·2NaCl·2H2O, and a solid solution series [Cs1–x(Na·H2O)x]Cl (x in [Cs1–x(Na·H2O)x]Cl changed from 0 to 0.43 (298.15 K), 0.39 (308.15 K), and 0.36 (318.15 K) at each temperature. With rising temperature, the crystallization area of NaCl increased, but CsCl·2NaCl·2H2O and [Cs1–x(Na·H2O)x]Cl decreased simultaneously. However, density and refractive index increased with increasing CsCl concentration.
中文翻译:

三元体系(CsCl + NaCl + H 2 O)在T =(298.15、308.15和318.15)K时的相图和理化性质
三元体系(CsCl + NaCl + H 2 O)的完整相图和理化性质分别在T =(298.15、308.15和318.15)K时确定。使用X射线衍射法和Schreinemaker的湿渣法验证固相。相图显示此三元系统是复杂类型。发现了三个结晶区域,分别对应于单一盐NaCl,化合物CsCl·2NaCl·2H 2 O和固溶体系列[Cs 1– x(Na·H 2 O)x ] Cl(x在[Cs 1– x中(Na·H 2 O)x在每个温度下,] Cl从0变为0.43(298.15 K),0.39(308.15 K)和0.36(318.15 K)。随着温度的升高,NaCl的结晶面积增加,但CsCl·2NaCl·2H 2 O和[Cs 1– x(Na·H 2 O)x ] Cl同时减少。但是,密度和折射率随CsCl浓度的增加而增加。
更新日期:2017-07-12
中文翻译:

三元体系(CsCl + NaCl + H 2 O)在T =(298.15、308.15和318.15)K时的相图和理化性质
三元体系(CsCl + NaCl + H 2 O)的完整相图和理化性质分别在T =(298.15、308.15和318.15)K时确定。使用X射线衍射法和Schreinemaker的湿渣法验证固相。相图显示此三元系统是复杂类型。发现了三个结晶区域,分别对应于单一盐NaCl,化合物CsCl·2NaCl·2H 2 O和固溶体系列[Cs 1– x(Na·H 2 O)x ] Cl(x在[Cs 1– x中(Na·H 2 O)x在每个温度下,] Cl从0变为0.43(298.15 K),0.39(308.15 K)和0.36(318.15 K)。随着温度的升高,NaCl的结晶面积增加,但CsCl·2NaCl·2H 2 O和[Cs 1– x(Na·H 2 O)x ] Cl同时减少。但是,密度和折射率随CsCl浓度的增加而增加。






























 京公网安备 11010802027423号
京公网安备 11010802027423号