当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enzymatic Reduction of Nicotinamide Biomimetic Cofactors Using an Engineered Glucose Dehydrogenase: Providing a Regeneration System for Artificial Cofactors
ACS Catalysis ( IF 11.3 ) Pub Date : 2017-07-11 00:00:00 , DOI: 10.1021/acscatal.7b00721 Claudia Nowak 1 , André Pick 1 , Petra Lommes 1 , Volker Sieber 1, 2
ACS Catalysis ( IF 11.3 ) Pub Date : 2017-07-11 00:00:00 , DOI: 10.1021/acscatal.7b00721 Claudia Nowak 1 , André Pick 1 , Petra Lommes 1 , Volker Sieber 1, 2
Affiliation
|
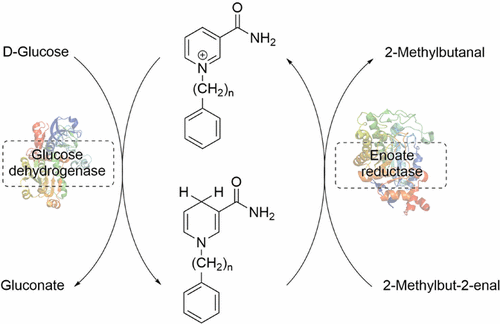
The increasing demand for chiral compounds supports the development of enzymatic processes. Dehydrogenases are often the enzymes of choice due to their high enantioselectivity combined with broad substrate acceptance. However, their requirement on costly NAD(P)/H as cofactor has sparked interest in the development of biomimetic derivatives that are easy to synthesize and, therefore, less expensive. Until now, few reactions with biomimetics have been described and regeneration is limited to nonenzymatic means, which are not suitable for incorporation and in situ approaches. Herein, we describe a regeneration enzyme, glucose dehydrogenase from Sulfolobus solfataricus (SsGDH), and demonstrate its activity with different biomimetics with the structure nicotinamide ring-alkyl chain-phenyl ring. Subsequent enzyme engineering resulted in the double mutant SsGDH Ile192Thr/Val306Ile, which had a 10-fold higher activity with one of the biomimetics compared with the wild-type enzyme. Using this engineered variant in combination with an enoate reductase from Thermus scotoductus resulted in the first enzyme-coupled regeneration process for biomimetic cofactor without ribonucleotide or ribonucleotide analogue and full conversion of 10 mM 2-methylbut-2-enal with 1-phenethyl-1,4-dihydropyridine-3-carboxamide as cofactor.
中文翻译:

使用工程葡萄糖脱氢酶的烟酰胺仿生辅酶的酶促还原:为人工辅因子提供再生系统。
对手性化合物的需求增加支持了酶促方法的发展。脱氢酶由于其高对映选择性和广泛的底物接受性,通常是选择的酶。然而,他们对昂贵的NAD(P)/ H作为辅因子的需求引起了人们对易于合成且因此价格便宜的仿生衍生物的开发兴趣。迄今为止,几乎没有描述与仿生物的反应,并且再生仅限于非酶促方法,该方法不适用于掺入和原位方法。在这里,我们描述了一种再生酶,从Sulfolobus solfataricus(Ss(GDH),并通过具有烟酰胺酰胺环-烷基链-苯环结构的不同仿生物证明其活性。随后的酶工程改造产生了双突变体Ss GDH Ile192Thr / Val306Ile,与野生型酶相比,其中一种仿生物的活性高10倍。将此工程化变体与Thermus scotoductus的烯酸还原酶结合使用,可实现不带核糖核苷酸或核糖核苷酸类似物的仿生辅因子的第一个酶偶联再生过程,以及10 mM 2-甲基丁-2-烯醛与1-phenethyl-1的完全转化,以4-二氢吡啶-3-羧酰胺为辅因子。
更新日期:2017-07-11
中文翻译:

使用工程葡萄糖脱氢酶的烟酰胺仿生辅酶的酶促还原:为人工辅因子提供再生系统。
对手性化合物的需求增加支持了酶促方法的发展。脱氢酶由于其高对映选择性和广泛的底物接受性,通常是选择的酶。然而,他们对昂贵的NAD(P)/ H作为辅因子的需求引起了人们对易于合成且因此价格便宜的仿生衍生物的开发兴趣。迄今为止,几乎没有描述与仿生物的反应,并且再生仅限于非酶促方法,该方法不适用于掺入和原位方法。在这里,我们描述了一种再生酶,从Sulfolobus solfataricus(Ss(GDH),并通过具有烟酰胺酰胺环-烷基链-苯环结构的不同仿生物证明其活性。随后的酶工程改造产生了双突变体Ss GDH Ile192Thr / Val306Ile,与野生型酶相比,其中一种仿生物的活性高10倍。将此工程化变体与Thermus scotoductus的烯酸还原酶结合使用,可实现不带核糖核苷酸或核糖核苷酸类似物的仿生辅因子的第一个酶偶联再生过程,以及10 mM 2-甲基丁-2-烯醛与1-phenethyl-1的完全转化,以4-二氢吡啶-3-羧酰胺为辅因子。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号