当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Effect of Promoters on Manganese-Containing Mixed Metal Oxides for Oxidative Dehydrogenation of Ethane via a Cyclic Redox Scheme
ACS Catalysis ( IF 11.3 ) Pub Date : 2017-07-10 00:00:00 , DOI: 10.1021/acscatal.7b02004 Seif Yusuf 1 , Luke M. Neal 1 , Fanxing Li 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2017-07-10 00:00:00 , DOI: 10.1021/acscatal.7b02004 Seif Yusuf 1 , Luke M. Neal 1 , Fanxing Li 1
Affiliation
|
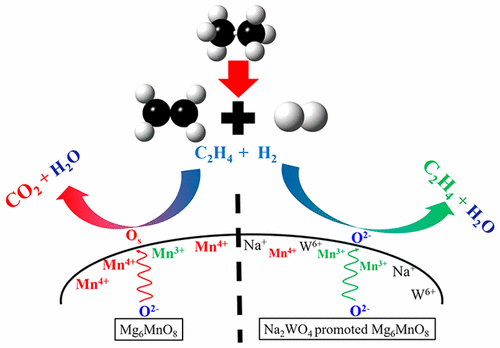
Ethylene is an important building block in the chemical industry; state of the art ethylene production (steam cracking) has multiple drawbacks, including high energy consumption, coke formation, and significant CO2 and NOx emissions. We propose a chemical looping oxidative dehydrogenation (CL-ODH) process to convert ethane into ethylene in a two-step, cyclic redox scheme. In this process, lattice oxygen in a metal oxide based redox catalyst is used to combust the hydrogen formed in ethane dehydrogenation, thereby enhancing ethylene formation while retarding coke formation. The oxygen-deprived redox catalyst is subsequently regenerated with air, releasing heat to balance the overall heat requirement. CL-ODH can realize a reduction of over 80% in primary energy consumption and pollutant emissions. The key to this process is an efficient redox catalyst with high selectivity and facile oxygen transport. Previously we determined that oxides with an Mg6MnO8 structure allow high lattice oxygen mobility and satisfactory oxygen-carrying capacity for the proposed redox reactions. However, unpromoted Mg6MnO8 exhibits poor ethylene selectivity, producing primarily CO2. In the current study, we examine the effects of various sodium-containing promoters on Mg6MnO8 CL-ODH activity and mechanism. Sodium tungstate promoted Mg6MnO8 was the most effective redox catalyst, showing an ethylene selectivity of 89.2% and yield of 68.2%, a significant improvement of thermal cracking (38.9% yield). Temperature-programmed reaction (TPR) experiments indicate that the reaction proceeds via gas-phase ethane thermal cracking in parallel with selective hydrogen combustion on the redox catalyst surface. XPS analysis indicates that the decreased ethane/ethylene oxidation activity on the sodium tungstate promoted redox catalysts results from the suppression of near-surface Mn4+. This is due to a combination of decreased surface manganese content and reduction in average Mn oxidation state. The suppression of Mn4+ results in a decrease in electrophilic surface oxygen species, inhibition of ethylene combustion, and enhanced ethylene yield.
中文翻译:

促进剂对含锰的混合金属氧化物对乙烷氧化还原脱氢的影响(循环氧化还原法)
乙烯是化学工业的重要组成部分。乙烯生产(蒸汽裂解)的技术水平具有多个缺点,包括高能耗,焦炭形成以及大量的CO 2和NO x排放。我们提出了一种化学环氧化脱氢(CL-ODH)工艺,可通过两步循环氧化还原方案将乙烷转化为乙烯。在该方法中,使用基于金属氧化物的氧化还原催化剂中的晶格氧来燃烧在乙烷脱氢中形成的氢,从而在阻止焦炭形成的同时增强乙烯的形成。缺氧的氧化还原催化剂随后与空气再生,释放热量以平衡总热量需求。CL-ODH可以减少一次能源消耗和污染物排放超过80%。该方法的关键是高效的氧化还原催化剂,具有高选择性和便捷的氧气传输能力。先前我们确定氧化物与Mg 6 MnO 8所提出的氧化还原反应的结构允许高的晶格氧迁移率和令人满意的载氧能力。然而,未促进的Mg 6 MnO 8表现出差的乙烯选择性,主要产生CO 2。在当前的研究中,我们检查了各种含钠促进剂对Mg 6 MnO 8 CL-ODH活性和机理的影响。钨酸钠促进的Mg 6 MnO 8是最有效的氧化还原催化剂,显示出89.2%的乙烯选择性和68.2%的产率,热裂解效果显着提高(产率38.9%)。程序升温反应(TPR)实验表明,该反应通过气相乙烷热裂化与氧化还原催化剂表面的选择性氢燃烧并行进行。XPS分析表明,在钨酸钠促进的氧化还原催化剂上乙烷/乙烯氧化活性的降低是由于抑制了近表面Mn 4+引起的。这是由于降低了表面锰含量和降低了平均Mn氧化态的结果。Mn 4+的抑制 导致亲电子表面氧种类的减少,乙烯燃烧的抑制和乙烯收率的提高。
更新日期:2017-07-11
中文翻译:

促进剂对含锰的混合金属氧化物对乙烷氧化还原脱氢的影响(循环氧化还原法)
乙烯是化学工业的重要组成部分。乙烯生产(蒸汽裂解)的技术水平具有多个缺点,包括高能耗,焦炭形成以及大量的CO 2和NO x排放。我们提出了一种化学环氧化脱氢(CL-ODH)工艺,可通过两步循环氧化还原方案将乙烷转化为乙烯。在该方法中,使用基于金属氧化物的氧化还原催化剂中的晶格氧来燃烧在乙烷脱氢中形成的氢,从而在阻止焦炭形成的同时增强乙烯的形成。缺氧的氧化还原催化剂随后与空气再生,释放热量以平衡总热量需求。CL-ODH可以减少一次能源消耗和污染物排放超过80%。该方法的关键是高效的氧化还原催化剂,具有高选择性和便捷的氧气传输能力。先前我们确定氧化物与Mg 6 MnO 8所提出的氧化还原反应的结构允许高的晶格氧迁移率和令人满意的载氧能力。然而,未促进的Mg 6 MnO 8表现出差的乙烯选择性,主要产生CO 2。在当前的研究中,我们检查了各种含钠促进剂对Mg 6 MnO 8 CL-ODH活性和机理的影响。钨酸钠促进的Mg 6 MnO 8是最有效的氧化还原催化剂,显示出89.2%的乙烯选择性和68.2%的产率,热裂解效果显着提高(产率38.9%)。程序升温反应(TPR)实验表明,该反应通过气相乙烷热裂化与氧化还原催化剂表面的选择性氢燃烧并行进行。XPS分析表明,在钨酸钠促进的氧化还原催化剂上乙烷/乙烯氧化活性的降低是由于抑制了近表面Mn 4+引起的。这是由于降低了表面锰含量和降低了平均Mn氧化态的结果。Mn 4+的抑制 导致亲电子表面氧种类的减少,乙烯燃烧的抑制和乙烯收率的提高。











































 京公网安备 11010802027423号
京公网安备 11010802027423号