当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Engineering the Aromaticity of Cationic Helical Polypeptides toward “Self-Activated” DNA/siRNA Delivery
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2017-07-10 00:00:00 , DOI: 10.1021/acsami.7b08534 Fangfang Li 1 , Yongjuan Li 1 , Zhuchao Zhou 2 , Shixian Lv 1 , Qiurong Deng 1 , Xin Xu 1 , Lichen Yin 1
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2017-07-10 00:00:00 , DOI: 10.1021/acsami.7b08534 Fangfang Li 1 , Yongjuan Li 1 , Zhuchao Zhou 2 , Shixian Lv 1 , Qiurong Deng 1 , Xin Xu 1 , Lichen Yin 1
Affiliation
|
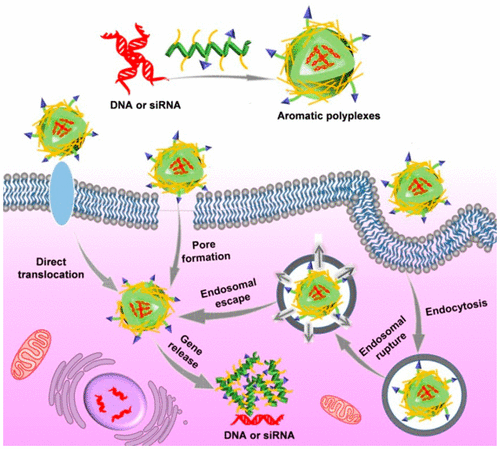
The development of potent yet nontoxic membrane-penetrating materials is in high demand for effective intracellular gene delivery. We have recently developed α-helical polypeptides which afford potent membrane activities to facilitate intracellular DNA delivery via both endocytosis and the nonendocytic “pore formation” mechanism. Endocytosis will cause endosomal entrapment of the DNA cargo, while excessive “pore formation” would cause appreciable cytotoxicity. Additionally, helical polypeptides with stiff, rodlike structure suffer from low siRNA binding affinity. To address such critical issues, we herein incorporated various aromatic domains (benzyl, naphthyl, biphenyl, anthryl, and pyrenyl) into the side-chain terminals of guanidine-rich, helical polypeptides, wherein the flat-rigid shape, π-electronic structures of aromatic motifs “self-activated” the membrane-penetrating capabilities of polypeptides to promote intracellular gene delivery. Benzyl (Bn)- and naphthyl (Naph)-modified polypeptides demonstrated the highest DNA uptake level that outperformed the unmodified polypeptide, P2, by ∼4 fold. More importantly, compared with P2, Bn- and Naph-modified polypeptides allowed more DNA cargos to be internalized via the nonendocytic pathway, which significantly bypassed the endosomal entrapment and accordingly enhanced the transfection efficiency by up to 42 fold, outperforming PEI 25k as the commercial reagent by 3–4 orders of magnitude. The aromatic modification also improved the siRNA condensation capability of polypeptides, achieving notably enhanced gene-silencing efficiency against tumor necrosis factor-α to treat acute hepatic inflammation. Furthermore, we revealed that aromaticity-augmented membrane activity was accompanied by comparable or even significantly reduced “pore formation” capability, thus leading to diminished cytotoxicity at high concentrations. This study therefore provides a promising approach to manipulate the membrane activities and penetration mechanisms of polycations, which overcomes the multiple critical barriers preventing effective and safe gene delivery.
中文翻译:

设计阳离子螺旋多肽对“自激活” DNA / siRNA传递的芳香性
对于有效的细胞内基因递送,迫切需要开发有效而无毒的膜穿透材料。我们最近开发了α-螺旋多肽,该多肽具有强大的膜活性,可通过内吞作用和非内吞性“孔形成”机制促进细胞内DNA的递送。内吞作用将引起DNA货物的内体包裹,而过多的“孔形成”将引起明显的细胞毒性。另外,具有刚性,棒状结构的螺旋多肽具有低的siRNA结合亲和力。为了解决这些关键问题,我们在本文中将各种芳族结构域(苄基,萘基,联苯,蒽基和pyr基)并入富含胍基的螺旋状多肽的侧链末端,其中,平面刚性形状,芳香基序的π电子结构“自我激活”了多肽的膜穿透能力,以促进细胞内基因的传递。苄基(Bn)和萘基(Naph)修饰的多肽显示出最高的DNA吸收水平,比未修饰的多肽P2高约4倍。更重要的是,与P2相比,Bn和Naph修饰的多肽允许通过非内吞途径将更多的DNA货物内在化,从而显着绕过了内体包裹,从而将转染效率提高了42倍,超过了PEI 25k试剂量降低3-4个数量级。芳香修饰还改善了多肽的siRNA缩合能力,显着提高了针对肿瘤坏死因子-α的基因沉默效率,以治疗急性肝炎。此外,我们发现芳香性增强的膜活性伴随着相当或什至显着降低的“孔形成”能力,从而导致高浓度时细胞毒性的降低。因此,这项研究提供了一种有前途的方法来操纵膜活性和阳离子的渗透机制,克服了阻止有效和安全的基因传递的多个关键障碍。
更新日期:2017-07-11
中文翻译:

设计阳离子螺旋多肽对“自激活” DNA / siRNA传递的芳香性
对于有效的细胞内基因递送,迫切需要开发有效而无毒的膜穿透材料。我们最近开发了α-螺旋多肽,该多肽具有强大的膜活性,可通过内吞作用和非内吞性“孔形成”机制促进细胞内DNA的递送。内吞作用将引起DNA货物的内体包裹,而过多的“孔形成”将引起明显的细胞毒性。另外,具有刚性,棒状结构的螺旋多肽具有低的siRNA结合亲和力。为了解决这些关键问题,我们在本文中将各种芳族结构域(苄基,萘基,联苯,蒽基和pyr基)并入富含胍基的螺旋状多肽的侧链末端,其中,平面刚性形状,芳香基序的π电子结构“自我激活”了多肽的膜穿透能力,以促进细胞内基因的传递。苄基(Bn)和萘基(Naph)修饰的多肽显示出最高的DNA吸收水平,比未修饰的多肽P2高约4倍。更重要的是,与P2相比,Bn和Naph修饰的多肽允许通过非内吞途径将更多的DNA货物内在化,从而显着绕过了内体包裹,从而将转染效率提高了42倍,超过了PEI 25k试剂量降低3-4个数量级。芳香修饰还改善了多肽的siRNA缩合能力,显着提高了针对肿瘤坏死因子-α的基因沉默效率,以治疗急性肝炎。此外,我们发现芳香性增强的膜活性伴随着相当或什至显着降低的“孔形成”能力,从而导致高浓度时细胞毒性的降低。因此,这项研究提供了一种有前途的方法来操纵膜活性和阳离子的渗透机制,克服了阻止有效和安全的基因传递的多个关键障碍。































 京公网安备 11010802027423号
京公网安备 11010802027423号