Tetrahedron ( IF 2.1 ) Pub Date : 2017-07-08 , DOI: 10.1016/j.tet.2017.07.009 Yu-Hsin Chen , Juan-Cheng Yang , Mei-Chin Lu , Ching-Feng Weng , Yin-Di Su , Jimmy Kuo , Yang-Chang Wu , Ping-Jyun Sung
|
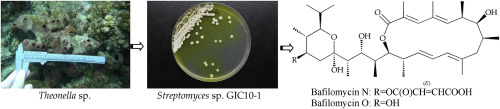
Two novel 16-membered tetraene marcolides, bafilomycins N (1) and O (2), along with two known analogues, JBIR-100 (3) and bafilomycin K (4) were produced from isolate GIC10-1, a Streptomyces sp. strain that was originally cloned from bacterial communities associated with marine sponge Theonella sp. The structures of new bafilomycins 1 and 2 were established by using spectroscopic methods and comparing the spectroscopic data with those of known related metabolites. Compounds 1 and 2 were proven to be the first 14-methylbafilomycins to be isolated. Cytotoxicity of these compounds toward various cancer cell lines also is described.
中文翻译:

Bafilomycins N和O,由链霉菌属(Streptomyces sp。)产生的新型细胞毒性bafilomycin类似物。从海洋海绵Theonella sp。分离的GIC10-1 。
从分离株GIC10-1(链霉菌属)中产生了两个新颖的16元四烯变质杆菌,bafilomycins N(1)和O(2)以及两种已知类似物JBIR-100(3)和bafilomycin K(4)。菌株最初是从与海洋海绵Theonella sp。相关的细菌群落中克隆的。通过使用光谱学方法,并将光谱数据与已知相关代谢物的光谱数据进行比较,来建立新的bafilomycins 1和2的结构。化合物1和2被证明是第一个被分离的14-甲基bafilomycins。还描述了这些化合物对各种癌细胞系的细胞毒性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号