当前位置:
X-MOL 学术
›
Tetrahedron Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
An intramolecular para-phenolic allylation free radical cyclization strategy for the synthesis of alkaloids and terpenes with spiro[4.5]decane architectures
Tetrahedron Letters ( IF 1.5 ) Pub Date : 2017-07-08 , DOI: 10.1016/j.tetlet.2017.07.003 Matthew G. Donahue , Nicholas G. Jentsch , Erin C. Realini
中文翻译:

螺内[4.5]癸烷结构合成生物碱和萜烯的分子内对-苯酚烯丙基化自由基环化策略
更新日期:2017-07-08
Tetrahedron Letters ( IF 1.5 ) Pub Date : 2017-07-08 , DOI: 10.1016/j.tetlet.2017.07.003 Matthew G. Donahue , Nicholas G. Jentsch , Erin C. Realini
|
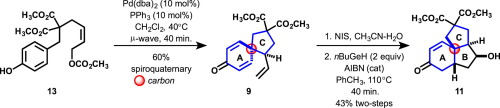
A Tsuji-Trost variant of the Winstein-Masamune reaction has been investigated for the synthesis of the AC spirocyclic ring system 9 bearing a quaternary carbon found in the fawcettimine type Lycopodium alkaloids magellanine 1 and lycojaponicumin B 2 and cyclopiane diterpenes such as conidiogenone 3. Annulation of the B ring for the synthesis of tricyclic ABC cores was demonstrated utilizing a 5-exo-trig free radical cyclization of a primary carbon radical onto a cyclohexadienone generated with tri-n-butylgermanium hydride (9 → 11).
中文翻译:

螺内[4.5]癸烷结构合成生物碱和萜烯的分子内对-苯酚烯丙基化自由基环化策略
该温斯腾-正宗反应的辻-特罗斯特变体已被研究用于AC螺环环系的合成9轴承的季碳在fawcettimine类型发现石松生物碱magellanine 1和lycojaponicumin乙2和cyclopiane双萜如conidiogenone 3。B环的环用于合成三环ABC芯证实利用5-外型-trig自由基伯碳基团环化到用三-产生的环己二烯酮Ñ -butylgermanium氢化物(9 → 11)。































 京公网安备 11010802027423号
京公网安备 11010802027423号