当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Low-Temperature CO Oxidation over Combustion Made Fe- and Cr-Doped Co3O4 Catalysts: Role of Dopant’s Nature toward Achieving Superior Catalytic Activity and Stability
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2017-07-07 00:00:00 , DOI: 10.1021/acs.jpcc.7b04348 Tinku Baidya 1 , Toru Murayama 2 , Parthasarathi Bera 3 , Olga V. Safonova 4 , Patrick Steiger 4 , Nirmal Kumar Katiyar 5 , Krishanu Biswas 5 , Masatake Haruta 2
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2017-07-07 00:00:00 , DOI: 10.1021/acs.jpcc.7b04348 Tinku Baidya 1 , Toru Murayama 2 , Parthasarathi Bera 3 , Olga V. Safonova 4 , Patrick Steiger 4 , Nirmal Kumar Katiyar 5 , Krishanu Biswas 5 , Masatake Haruta 2
Affiliation
|
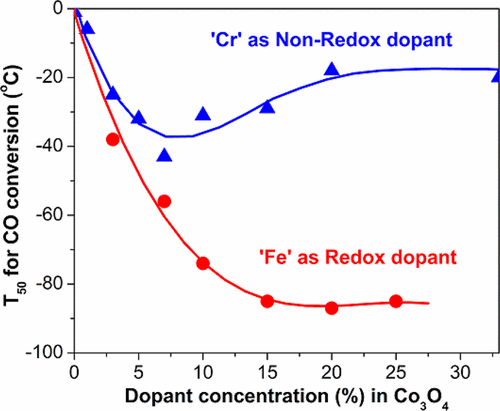
Co3O4 with a spinel structure shows unique activity for CO oxidation at low temperature under dry conditions; however the active surface is not very stable. In this study, two series of Fe- and Cr-doped Co3O4 catalysts were prepared by a single-step solution combustion technique. Fe was chosen because of its redox activity corresponding to the Fe2+/Fe3+ redox couple and compared to Cr, which is mainly stable in the Cr3+ state. The catalytic activity of new materials for low-temperature CO oxidation was correlated to the nature of the dopant. As a function of dopant concentration, the temperature corresponding to the 50% CO conversion (T50) demonstrated significant differences. The maximal activity was achieved for 15% Fe-doped Co3O4 with T50 of −85 °C and remained almost constant up to 25% Fe. In the case of Cr, the activity was observed to be maximum for 7% of Cr with T50 of −42 °C and significantly decreased for higher Cr loadings. Similarly, there was a contrasting behavior in catalyst stability too. 100% CO conversion was achieved below −60 °C for 15% Fe/Co3O4 catalyst and remained unchanged even after calcination at 600 °C. In contrast, Co3O4 or 15% Cr/Co3O4 catalysts strongly deactivated after the same treatment. These differences were correlated to the oxidation states, coordination numbers, the nature of surface planes, and the redox properties. We observed that both Cr and Fe were typically present in the +3 oxidation state, occupying octahedral sites in the spinel structure. The catalysts were mainly exposed to (111) and (220) planes on the surface. H2-TPR indicated clear differences in the redox activity of materials due to Fe and Cr substitutions. The reducibility of surface Co3+ species remained similar in all Fe-doped Co3O4 catalysts in contrast to nonreducible Cr-doped analogs, which shifted the reduction temperature to the higher values. As the Fe3+/Fe2+ redox couple partly substituted the Co3+/Co2+ redox couple in the spinel structure, similar bond strength of Fe–O keep redox activity of Co3+ species almost unchanged leading to higher activity and stability of Fe/Co3O4 catalysts for low-temperature CO oxidation. In contrast, nonreducible Cr3+ species characterized by strong Cr–O bond substituting active Co3+ sites can make the Cr/Co3O4 surface less active for CO oxidation.
中文翻译:

燃烧制成的Fe和Cr掺杂的Co 3 O 4催化剂上的低温CO氧化:掺杂剂的性质对实现优异的催化活性和稳定性的作用
具有尖晶石结构的Co 3 O 4在干燥条件下在低温下表现出独特的CO氧化活性;但是,活性表面不是很稳定。在这项研究中,通过单步固溶燃烧技术制备了两个系列的Fe和Cr掺杂的Co 3 O 4催化剂。选择Fe是因为其氧化还原活性对应于Fe 2+ / Fe 3+氧化还原对,并且与Cr相比,Cr主要在Cr 3+状态下稳定。新材料对低温CO氧化的催化活性与掺杂剂的性质有关。作为掺杂剂浓度的函数,温度对应于50%的CO转化率(T50)表现出显着差异。最大活性的15%Fe掺杂钴达到3 ö 4与Ť 50 -85℃,并几乎保持不变达25%的Fe。在Cr的情况下,在5%的T 50为-42°C时,发现Cr的活性最高,为7%,而在较高的Cr负载量下活性则显着降低。类似地,催化剂稳定性也有相反的行为。15%Fe / Co 3 O 4催化剂在-60°C以下达到100%CO转化率,即使在600°C煅烧后也保持不变。相反,Co 3 O 4或15%Cr / Co 3 O 4相同处理后,催化剂会严重失活。这些差异与氧化态,配位数,表面平面的性质和氧化还原特性有关。我们观察到,Cr和Fe都通常以+3氧化态存在,占据尖晶石结构中的八面体位置。催化剂主要暴露在表面的(111)和(220)平面上。H 2 -TPR表明由于Fe和Cr取代,材料的氧化还原活性明显不同。与所有不可还原的Cr掺杂类似物相比,在所有Fe掺杂的Co 3 O 4催化剂中,表面Co 3+物种的可还原性保持相似,从而将还原温度移至更高的值。作为Fe 3+/ Fe 2+氧化还原对在尖晶石结构中部分取代了Co 3+ / Co 2+氧化还原对,相似的Fe–O键强度使Co 3+的氧化还原活性几乎保持不变,从而提高了Fe / Co的活性和稳定性3 O 4催化剂,用于低温CO氧化。相比之下,不可还原的Cr 3+物种具有强大的Cr-O键取代了活性Co 3+位点,可以使Cr / Co 3 O 4表面对CO氧化的活性降低。
更新日期:2017-07-08
中文翻译:

燃烧制成的Fe和Cr掺杂的Co 3 O 4催化剂上的低温CO氧化:掺杂剂的性质对实现优异的催化活性和稳定性的作用
具有尖晶石结构的Co 3 O 4在干燥条件下在低温下表现出独特的CO氧化活性;但是,活性表面不是很稳定。在这项研究中,通过单步固溶燃烧技术制备了两个系列的Fe和Cr掺杂的Co 3 O 4催化剂。选择Fe是因为其氧化还原活性对应于Fe 2+ / Fe 3+氧化还原对,并且与Cr相比,Cr主要在Cr 3+状态下稳定。新材料对低温CO氧化的催化活性与掺杂剂的性质有关。作为掺杂剂浓度的函数,温度对应于50%的CO转化率(T50)表现出显着差异。最大活性的15%Fe掺杂钴达到3 ö 4与Ť 50 -85℃,并几乎保持不变达25%的Fe。在Cr的情况下,在5%的T 50为-42°C时,发现Cr的活性最高,为7%,而在较高的Cr负载量下活性则显着降低。类似地,催化剂稳定性也有相反的行为。15%Fe / Co 3 O 4催化剂在-60°C以下达到100%CO转化率,即使在600°C煅烧后也保持不变。相反,Co 3 O 4或15%Cr / Co 3 O 4相同处理后,催化剂会严重失活。这些差异与氧化态,配位数,表面平面的性质和氧化还原特性有关。我们观察到,Cr和Fe都通常以+3氧化态存在,占据尖晶石结构中的八面体位置。催化剂主要暴露在表面的(111)和(220)平面上。H 2 -TPR表明由于Fe和Cr取代,材料的氧化还原活性明显不同。与所有不可还原的Cr掺杂类似物相比,在所有Fe掺杂的Co 3 O 4催化剂中,表面Co 3+物种的可还原性保持相似,从而将还原温度移至更高的值。作为Fe 3+/ Fe 2+氧化还原对在尖晶石结构中部分取代了Co 3+ / Co 2+氧化还原对,相似的Fe–O键强度使Co 3+的氧化还原活性几乎保持不变,从而提高了Fe / Co的活性和稳定性3 O 4催化剂,用于低温CO氧化。相比之下,不可还原的Cr 3+物种具有强大的Cr-O键取代了活性Co 3+位点,可以使Cr / Co 3 O 4表面对CO氧化的活性降低。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号