当前位置:
X-MOL 学术
›
Adv. Funct. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
MUC1 Aptamer Targeted SERS Nanoprobes
Advanced Functional Materials ( IF 18.5 ) Pub Date : 2017-07-06 , DOI: 10.1002/adfm.201606632 Suchetan Pal 1 , Stefan Harmsen 1 , Anton Oseledchyk 1 , Hsiao-Ting Hsu 1 , Moritz F Kircher 2
Advanced Functional Materials ( IF 18.5 ) Pub Date : 2017-07-06 , DOI: 10.1002/adfm.201606632 Suchetan Pal 1 , Stefan Harmsen 1 , Anton Oseledchyk 1 , Hsiao-Ting Hsu 1 , Moritz F Kircher 2
Affiliation
|
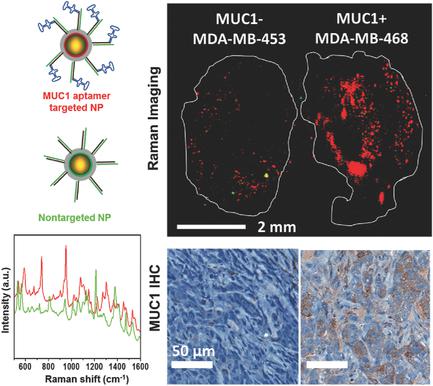
Recently, surface‐enhanced Raman scattering (SERS) nanoprobes (NPs) have shown promise in the field of cancer imaging due to their unparalleled signal specificity and high sensitivity. This study reports the development of a DNA aptamer targeted SERS NP. Recently, aptamers are being investigated as a viable alternative to more traditional antibody targeting due to their low immunogenicity and low cost of production. A strategy is developed to functionalize SERS NPs with DNA aptamers, which target Mucin1 (MUC1) in human breast cancer (BC). Thorough in vitro characterization studies demonstrate excellent serum stability and specific binding of the targeted NPs to MUC1. In order to test their in vivo targeting capability, MUC1‐targeted SERS NPs are coinjected with nontargeted or blocked MUC1‐targeted SERS NPs in BC xenograft mouse models. A two‐tumor mouse model with differential expression of MUC1 (MDA‐MB‐468 and MDA‐MB‐453) is used to control for active versus passive targeting in the same animals. The results show that the targeted SERS NPs home to the tumors via active targeting of MUC1, with low levels of passive targeting. This strategy is expected to be an advantageous alternative to antibody‐based targeting and useful for targeted imaging of tumor extent, progression, and therapeutic response.
中文翻译:

MUC1 适体靶向 SERS 纳米探针
最近,表面增强拉曼散射(SERS)纳米探针(NP)由于其无与伦比的信号特异性和高灵敏度而在癌症成像领域显示出前景。本研究报告了针对 SERS NP 的 DNA 适体的开发。最近,由于适体的免疫原性低和生产成本低,人们正在研究适体作为更传统的抗体靶向的可行替代品。开发了一种利用 DNA 适体对 SERS 纳米颗粒进行功能化的策略,该适体针对人类乳腺癌 (BC) 中的粘蛋白 1 (MUC1)。彻底的体外表征研究表明,靶向 NP 具有出色的血清稳定性和与 MUC1 的特异性结合。为了测试它们的体内靶向能力,将MUC1靶向SERS NP与非靶向或封闭的MUC1靶向SERS NP共同注射到BC异种移植小鼠模型中。使用具有 MUC1 差异表达的双肿瘤小鼠模型(MDA-MB-468 和 MDA-MB-453)来控制同一动物的主动靶向与被动靶向。结果表明,靶向SERS NPs通过主动靶向MUC1而归巢于肿瘤,而被动靶向水平较低。该策略有望成为基于抗体的靶向的有利替代方案,并且可用于肿瘤范围、进展和治疗反应的靶向成像。
更新日期:2017-07-06
中文翻译:

MUC1 适体靶向 SERS 纳米探针
最近,表面增强拉曼散射(SERS)纳米探针(NP)由于其无与伦比的信号特异性和高灵敏度而在癌症成像领域显示出前景。本研究报告了针对 SERS NP 的 DNA 适体的开发。最近,由于适体的免疫原性低和生产成本低,人们正在研究适体作为更传统的抗体靶向的可行替代品。开发了一种利用 DNA 适体对 SERS 纳米颗粒进行功能化的策略,该适体针对人类乳腺癌 (BC) 中的粘蛋白 1 (MUC1)。彻底的体外表征研究表明,靶向 NP 具有出色的血清稳定性和与 MUC1 的特异性结合。为了测试它们的体内靶向能力,将MUC1靶向SERS NP与非靶向或封闭的MUC1靶向SERS NP共同注射到BC异种移植小鼠模型中。使用具有 MUC1 差异表达的双肿瘤小鼠模型(MDA-MB-468 和 MDA-MB-453)来控制同一动物的主动靶向与被动靶向。结果表明,靶向SERS NPs通过主动靶向MUC1而归巢于肿瘤,而被动靶向水平较低。该策略有望成为基于抗体的靶向的有利替代方案,并且可用于肿瘤范围、进展和治疗反应的靶向成像。































 京公网安备 11010802027423号
京公网安备 11010802027423号