当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enantiospecific Synthesis of ortho-Substituted Benzylic Boronic Esters by a 1,2-Metalate Rearrangement/1,3-Borotropic Shift Sequence
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-07-06 00:00:00 , DOI: 10.1021/jacs.7b05880 Stefan Aichhorn 1 , Raphael Bigler 1 , Eddie L. Myers 1 , Varinder K. Aggarwal 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-07-06 00:00:00 , DOI: 10.1021/jacs.7b05880 Stefan Aichhorn 1 , Raphael Bigler 1 , Eddie L. Myers 1 , Varinder K. Aggarwal 1
Affiliation
|
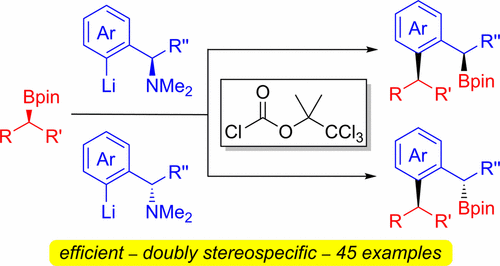
Coupling reactions between benzylamines and boronic esters have been investigated. ortho-Lithiated benzylamines react with boronic esters and a N-activator to afford ortho-substituted benzylic boronic esters with formal 1,1′-benzylidene insertion into the C–B bond. The reaction occurs by a SN2′ elimination and 1,2-metalate rearrangement of the N-activated boronate complex to afford a dearomatized intermediate, which undergoes a Lewis-acid catalyzed 1,3-borotropic shift to afford the boronic ester products in high yield and with excellent enantiospecificity. The use of enantioenriched α-substituted benzylamines gave the corresponding secondary boronic esters with high ee.
中文翻译:

通过1,2-金属重排/ 1,3-硼亲和移位序列对位取代的苄基硼酸酯的对映体合成
已经研究了苄胺和硼酸酯之间的偶联反应。邻-Lithiated苄胺与硼酸酯反应,并且一个Ñ -activator,得到邻位与正规1,1'-亚苄基取代的插入苄硼酸酯到C-B键。该反应通过S N 2'的消除和N活化的硼酸酯络合物的1,2-金属化物重排而得到脱芳香化的中间体,该中间体经过路易斯酸催化的1,3-硼向转变以得到硼酸酯产物。高收率和优异的对映体特异性。使用富含对映体的α-取代的苄胺得到相应的具有高ee的仲硼酸酯。
更新日期:2017-07-06
中文翻译:

通过1,2-金属重排/ 1,3-硼亲和移位序列对位取代的苄基硼酸酯的对映体合成
已经研究了苄胺和硼酸酯之间的偶联反应。邻-Lithiated苄胺与硼酸酯反应,并且一个Ñ -activator,得到邻位与正规1,1'-亚苄基取代的插入苄硼酸酯到C-B键。该反应通过S N 2'的消除和N活化的硼酸酯络合物的1,2-金属化物重排而得到脱芳香化的中间体,该中间体经过路易斯酸催化的1,3-硼向转变以得到硼酸酯产物。高收率和优异的对映体特异性。使用富含对映体的α-取代的苄胺得到相应的具有高ee的仲硼酸酯。































 京公网安备 11010802027423号
京公网安备 11010802027423号