Bioorganic & Medicinal Chemistry Letters ( IF 2.5 ) Pub Date : 2017-07-05 , DOI: 10.1016/j.bmcl.2017.06.078 Yoshihiro Usui , Fumiaki Uehara , Shinsuke Hiki , Kazutoshi Watanabe , Hiroshi Tanaka , Aya Shouda , Satoshi Yokoshima , Keiichi Aritomo , Takashi Adachi , Kenji Fukunaga , Shinji Sunada , Mika Nabeno , Ken-Ichi Saito , Jun-ichi Eguchi , Keiji Yamagami , Shouichi Asano , Shinji Tanaka , Satoshi Yuki , Narihiko Yoshii , Masatake Fujimura , Takashi Horikawa
|
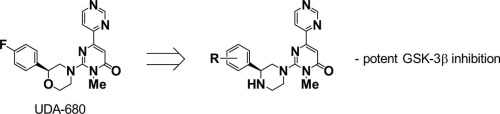
We herein describe the results of further evolution of glycogen synthase kinase (GSK)-3β inhibitors from our promising compounds containing a 2-phenylmorpholine moiety. Transformation of the morpholine moiety into a piperazine moiety resulted in potent GSK-3β inhibitors. SAR studies focused on the phenyl moiety revealed that a 4-fluoro-2-methoxy group afforded potent inhibitory activity toward GSK-3β. Based on docking studies, new hydrogen bonding between the nitrogen atom of the piperazine moiety and the oxygen atom of the main chain of Gln185 has been indicated, which may contribute to increased activity compared with that of the corresponding phenylmorpholine analogues. Effect of the stereochemistry of the phenylpiperazine moiety is also discussed.
中文翻译:

新型2-(3-苯基哌嗪-1-基)-嘧啶-4-酮作为糖原合酶激酶-3β抑制剂的发现
我们在本文中描述了糖原合酶激酶(GSK)-3β抑制剂从含有2-苯基吗啉部分的有希望的化合物中进一步进化的结果。吗啉部分转化为哌嗪部分会产生有效的GSK-3β抑制剂。专注于苯基部分的SAR研究表明,4-氟-2-甲氧基对GSK-3β具有有效的抑制活性。基于对接研究,已表明哌嗪部分的氮原子与Gln185主链的氧原子之间存在新的氢键,与相应的苯吗啉类似物相比,这可能有助于增加活性。还讨论了苯基哌嗪部分的立体化学作用。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号