当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Hydrolysis-Coupled Redox Reaction to 3D Cu/Fe3O4 Nanorod Array Electrodes for High-Performance Lithium-Ion Batteries
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2017-07-05 00:00:00 , DOI: 10.1021/acs.inorgchem.7b00112 Heyun Gu 1 , Yingmeng Zhang 1 , Mengqiu Huang 1 , Fei Chen 1 , Zeheng Yang 1 , Xiaoming Fan 1 , Sheng Li 1 , Weixin Zhang 1 , Shihe Yang 2 , Mei Li 3
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2017-07-05 00:00:00 , DOI: 10.1021/acs.inorgchem.7b00112 Heyun Gu 1 , Yingmeng Zhang 1 , Mengqiu Huang 1 , Fei Chen 1 , Zeheng Yang 1 , Xiaoming Fan 1 , Sheng Li 1 , Weixin Zhang 1 , Shihe Yang 2 , Mei Li 3
Affiliation
|
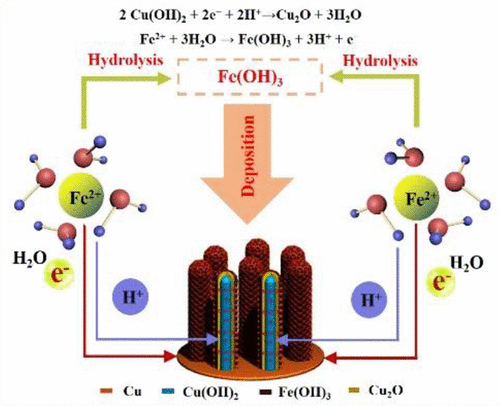
A facile hydrolysis-coupled redox (HCR) reaction followed by postheating reduction has been designed to prepare unique 3D Cu/Fe3O4 core–shell nanorod array anodes. Fe2+ ions from fresh FeSO4 solution have been hydrolyzed and oxidized to form an Fe(OH)3 shell on the surface of Cu(OH)2 nanorods; meanwhile the resulting acidic environment induces the reduction of Cu(OH)2 to Cu2O, which realizes an unusual redox reaction between Fe2+ ions and Cu(OH)2. The reaction procedure and thermodynamics possibility between Fe2+ ions and Cu(OH)2 nanorod arrays are discussed from the aspect of electrode potentials. After postheating reduction in Ar/H2, the obtained 3D architecture of Cu current collector serves as a stout support for the Fe3O4 shell to form nanorod array anodes without using any binders or conducting agents. The resulting highly stable core–shell structure facilitates rapid and high-throughput transport pathways for ions/electrons and allows better accommodation of volume change during the repeated lithiation/delithiation. Its application as anodes in combination with LiNi0.5Mn1.5O4 cathodes for full cells demonstrates superior rate capability, enhanced energy density, and long cycling life.
中文翻译:

高性能锂离子电池3D Cu / Fe 3 O 4纳米棒阵列电极的水解偶联氧化还原反应
设计了一种简单的水解偶联氧化还原(HCR)反应,然后进行后热还原反应,以制备独特的3D Cu / Fe 3 O 4核-壳纳米棒阵列阳极。新鲜的FeSO 4溶液中的Fe 2+离子经过水解和氧化,在Cu(OH)2纳米棒的表面形成Fe(OH)3壳;同时,所产生的酸性环境促使Cu(OH)2还原为Cu 2 O,从而实现了Fe 2+离子与Cu(OH)2之间异常的氧化还原反应。Fe 2+离子与Cu(OH)2的反应过程和热力学可能性从电极电位的角度讨论了纳米棒阵列。在Ar / H 2的后热还原之后,所获得的Cu集电器的3D结构可作为Fe 3 O 4壳的粗壮支撑物,以形成纳米棒阵列阳极,而无需使用任何粘合剂或导电剂。由此产生的高度稳定的核-壳结构促进了离子/电子的快速和高通量传输路径,并允许在重复锂化/脱锂过程中更好地适应体积变化。它与完整的电池NiNi 0.5 Mn 1.5 O 4阴极一起用作阳极,显示出出众的倍率能力,增强的能量密度和长循环寿命。
更新日期:2017-07-05
中文翻译:

高性能锂离子电池3D Cu / Fe 3 O 4纳米棒阵列电极的水解偶联氧化还原反应
设计了一种简单的水解偶联氧化还原(HCR)反应,然后进行后热还原反应,以制备独特的3D Cu / Fe 3 O 4核-壳纳米棒阵列阳极。新鲜的FeSO 4溶液中的Fe 2+离子经过水解和氧化,在Cu(OH)2纳米棒的表面形成Fe(OH)3壳;同时,所产生的酸性环境促使Cu(OH)2还原为Cu 2 O,从而实现了Fe 2+离子与Cu(OH)2之间异常的氧化还原反应。Fe 2+离子与Cu(OH)2的反应过程和热力学可能性从电极电位的角度讨论了纳米棒阵列。在Ar / H 2的后热还原之后,所获得的Cu集电器的3D结构可作为Fe 3 O 4壳的粗壮支撑物,以形成纳米棒阵列阳极,而无需使用任何粘合剂或导电剂。由此产生的高度稳定的核-壳结构促进了离子/电子的快速和高通量传输路径,并允许在重复锂化/脱锂过程中更好地适应体积变化。它与完整的电池NiNi 0.5 Mn 1.5 O 4阴极一起用作阳极,显示出出众的倍率能力,增强的能量密度和长循环寿命。

































 京公网安备 11010802027423号
京公网安备 11010802027423号