Bioorganic & Medicinal Chemistry Letters ( IF 2.5 ) Pub Date : 2017-07-05 , DOI: 10.1016/j.bmcl.2017.06.077 Toshiyuki Kohara , Kazuki Nakayama , Kazutoshi Watanabe , Shin-ichi Kusaka , Daiki Sakai , Hiroshi Tanaka , Kenji Fukunaga , Shinji Sunada , Mika Nabeno , Ken-Ichi Saito , Jun-ichi Eguchi , Akiko Mori , Shinji Tanaka , Tomoko Bessho , Keiko Takiguchi-Hayashi , Takashi Horikawa
|
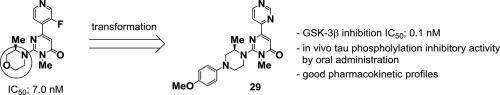
We herein describe the results of further evolution of glycogen synthase kinase (GSK)-3β inhibitors from our promising compounds containing a 3-methylmorpholine moiety. Transformation of the morpholine moiety into a piperazine moiety resulted in potent GSK-3β inhibitors. SAR studies focused on the nitrogen atom of the piperazine moiety revealed that a phenyl group afforded potent inhibitory activity toward GSK-3β. Docking studies indicated that the phenyl group on the piperazine nitrogen atom and the methyl group on the piperazine make cation-π and CH-π interactions with GSK-3β respectively. 4-Methoxyphenyl analogue 29 showed most potent inhibitory activity toward GSK-3β with good in vitro and in vivo pharmacokinetic profiles, and 29 demonstrated a significant decrease in tau phosphorylation after oral administration in mice.
中文翻译:

新型2-(4-芳基-2-甲基哌嗪-1-基)-嘧啶-4-酮作为糖原合酶激酶-3β抑制剂的发现
我们在本文中描述了糖原合酶激酶(GSK)-3β抑制剂从含有3-甲基吗啉部分的有希望的化合物中进一步进化的结果。吗啉部分转化为哌嗪部分会产生有效的GSK-3β抑制剂。SAR研究集中于哌嗪部分的氮原子,发现苯基对GSK-3β具有强抑制作用。对接研究表明,哌嗪氮原子上的苯基和哌嗪上的甲基分别与GSK-3β发生阳离子-π和CH-π相互作用。4-甲氧基苯基类似物29对GSK-3β表现出最强的抑制活性,具有良好的体内和体外药代动力学特性,而29 在小鼠口服后证明tau磷酸化显着降低。































 京公网安备 11010802027423号
京公网安备 11010802027423号