当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Solid-state NMR as a probe of anion binding: molecular dynamics and associations in a [5]polynorbornane bisurea host complexed with terephthalate†
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2015-07-28 00:00:00 , DOI: 10.1039/c5cp03628c Aditya Rawal 1, 2, 3, 4 , James M. Hook 1, 2, 3, 4 , Ryan N. Robson 4, 5, 6, 7, 8 , Daniel Gunzelmann 4, 7, 8, 9 , Frederick M. Pfeffer 4, 5, 6, 7, 8 , Luke A. O'Dell 4, 7, 8, 9
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2015-07-28 00:00:00 , DOI: 10.1039/c5cp03628c Aditya Rawal 1, 2, 3, 4 , James M. Hook 1, 2, 3, 4 , Ryan N. Robson 4, 5, 6, 7, 8 , Daniel Gunzelmann 4, 7, 8, 9 , Frederick M. Pfeffer 4, 5, 6, 7, 8 , Luke A. O'Dell 4, 7, 8, 9
Affiliation
|
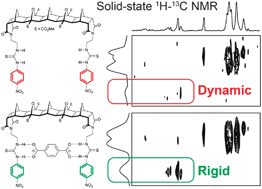
A range of solid-state NMR techniques is used to characterise a molecular host:guest complex consisting of a [5]polynorbornane bisurea host binding a terephthalate dianion guest. Detailed information is obtained on the molecular dynamics and associations from the point of view of both the host and guest molecules. The formation of the complex in the solid state is confirmed using 1H 2D exchange NMR, and the 180° flipping of the 2H-labelled terephthalate guest and its eventual expulsion from the complex at elevated temperatures are quantified using variable-temperature 2H spin-echo experiments. Two-dimensional 1H–13C HETCOR spectra obtained under fast magic angle spinning conditions (60 kHz) show a high resolution despite the poor crystallinity of the solid complex, and clearly reveal changes in the rigidity of the host molecule when complexed. Short-range intra- and intermolecular 1H–1H proximities are also detected using 2D SQ–DQ correlation methods, providing insight into the molecular packing in the solid phase.
中文翻译:

固态NMR作为阴离子结合的探针:与对苯二甲酸酯络合的[5]聚降冰片烷双脲主体中的分子动力学和缔合†
一系列的固态NMR技术用于表征分子主体:客体复合物,该复合物由与对苯二甲酸二阴离子客体结合的[5]聚降冰片烷双脲主体组成。从宿主分子和客体分子的角度都可以获得有关分子动力学和缔合的详细信息。使用1 H 2D交换NMR确认了固态复合物的形成,并使用可变温度的2 H自旋定量了2 H标记的对苯二甲酸对映体180°翻转以及在高温下最终从复合物中的排出。-回声实验。二维1 H– 13尽管固体配合物的结晶度较差,但在快速魔角旋转条件下(60 kHz)获得的C HETCOR光谱仍显示出较高的分辨率,并且清楚地揭示了配合时主体分子刚性的变化。还可以使用2D SQ-DQ相关方法检测分子内和分子间1 H- 1 H的近距离,从而深入了解固相中的分子堆积。
更新日期:2015-07-28
中文翻译:

固态NMR作为阴离子结合的探针:与对苯二甲酸酯络合的[5]聚降冰片烷双脲主体中的分子动力学和缔合†
一系列的固态NMR技术用于表征分子主体:客体复合物,该复合物由与对苯二甲酸二阴离子客体结合的[5]聚降冰片烷双脲主体组成。从宿主分子和客体分子的角度都可以获得有关分子动力学和缔合的详细信息。使用1 H 2D交换NMR确认了固态复合物的形成,并使用可变温度的2 H自旋定量了2 H标记的对苯二甲酸对映体180°翻转以及在高温下最终从复合物中的排出。-回声实验。二维1 H– 13尽管固体配合物的结晶度较差,但在快速魔角旋转条件下(60 kHz)获得的C HETCOR光谱仍显示出较高的分辨率,并且清楚地揭示了配合时主体分子刚性的变化。还可以使用2D SQ-DQ相关方法检测分子内和分子间1 H- 1 H的近距离,从而深入了解固相中的分子堆积。































 京公网安备 11010802027423号
京公网安备 11010802027423号