Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Syncytiotrophoblast derived extracellular vesicles transfer functional placental miRNAs to primary human endothelial cells.
Scientific Reports ( IF 3.8 ) Pub Date : 2017-07-04 , DOI: 10.1038/s41598-017-04468-0 Tina Cronqvist , Dionne Tannetta , Matthias Mörgelin , Mattias Belting , Ian Sargent , Mary Familari , Stefan R. Hansson
Scientific Reports ( IF 3.8 ) Pub Date : 2017-07-04 , DOI: 10.1038/s41598-017-04468-0 Tina Cronqvist , Dionne Tannetta , Matthias Mörgelin , Mattias Belting , Ian Sargent , Mary Familari , Stefan R. Hansson
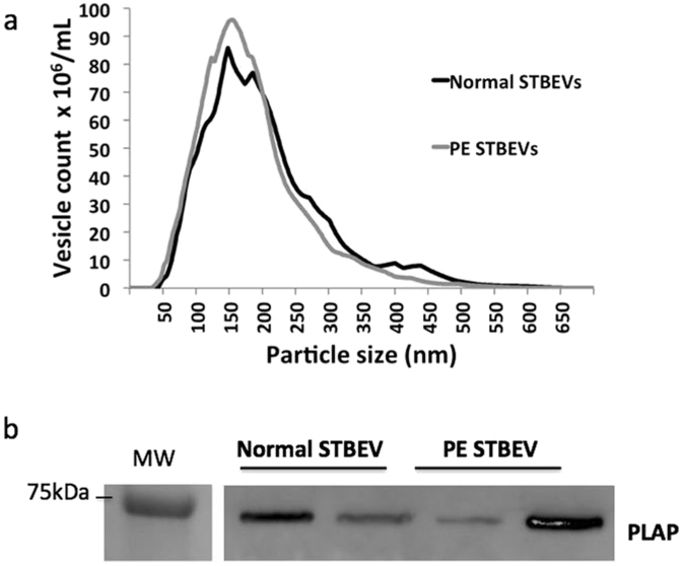
|
During the pregnancy associated syndrome preeclampsia (PE), there is increased release of placental syncytiotrophoblast extracellular vesicles (STBEVs) and free foetal haemoglobin (HbF) into the maternal circulation. In the present study we investigated the uptake of normal and PE STBEVs by primary human coronary artery endothelial cells (HCAEC) and the effects of free HbF on this uptake. Our results show internalization of STBEVs into primary HCAEC, and transfer of placenta specific miRNAs from STBEVs into the endoplasmic reticulum and mitochondria of these recipient cells. Further, the transferred miRNAs were functional, causing a down regulation of specific target genes, including the PE associated gene fms related tyrosine kinase 1 (FLT1). When co-treating normal STBEVs with HbF, the miRNA deposition is altered from the mitochondria to the ER and the cell membrane becomes ruffled, as was also seen with PE STBEVs. These findings suggest that STBEVs may cause endothelial damage and contribute to the endothelial dysfunction typical for PE. The miRNA mediated effects on gene expression may contribute to the oxidative and endoplasmic reticulum stress described in PE, as well as endothelial reprogramming that may underlay the increased risk of cardiovascular disease reported for women with PE later in life.
中文翻译:

合体滋养层细胞衍生的细胞外囊泡将功能性胎盘miRNA转移至原代人内皮细胞。
在妊娠相关子痫前期(PE)期间,胎盘合体滋养层细胞外囊泡(STBEVs)和游离胎儿血红蛋白(HbF)释放到母体循环中的释放增加。在本研究中,我们调查了原代人冠状动脉内皮细胞(HCAEC)对正常STBEV和PE STBEV的摄取以及游离HbF对这种摄取的影响。我们的研究结果表明,STBEVs内在化进入原发性HCAEC,胎盘特异性miRNA从STBEVs转移到这些受体细胞的内质网和线粒体中。此外,转移的miRNA具有功能,导致特定靶基因的下调,包括PE相关基因fms相关酪氨酸激酶1(FLT1)。当将普通STBEV与HbF进行共处理时,从线粒体到内质网,miRNA的沉积发生了变化,细胞膜也变得褶皱了,这在PE STBEV中也可以看到。这些发现表明,STBEVs可能引起内皮损伤,并导致典型的PE内皮功能障碍。miRNA对基因表达的介导作用可能会导致PE中描述的氧化和内质网应激,以及内皮重编程,这可能会增加据报道在以后生活中患有PE的女性患心血管疾病的风险。
更新日期:2017-07-05
中文翻译:

合体滋养层细胞衍生的细胞外囊泡将功能性胎盘miRNA转移至原代人内皮细胞。
在妊娠相关子痫前期(PE)期间,胎盘合体滋养层细胞外囊泡(STBEVs)和游离胎儿血红蛋白(HbF)释放到母体循环中的释放增加。在本研究中,我们调查了原代人冠状动脉内皮细胞(HCAEC)对正常STBEV和PE STBEV的摄取以及游离HbF对这种摄取的影响。我们的研究结果表明,STBEVs内在化进入原发性HCAEC,胎盘特异性miRNA从STBEVs转移到这些受体细胞的内质网和线粒体中。此外,转移的miRNA具有功能,导致特定靶基因的下调,包括PE相关基因fms相关酪氨酸激酶1(FLT1)。当将普通STBEV与HbF进行共处理时,从线粒体到内质网,miRNA的沉积发生了变化,细胞膜也变得褶皱了,这在PE STBEV中也可以看到。这些发现表明,STBEVs可能引起内皮损伤,并导致典型的PE内皮功能障碍。miRNA对基因表达的介导作用可能会导致PE中描述的氧化和内质网应激,以及内皮重编程,这可能会增加据报道在以后生活中患有PE的女性患心血管疾病的风险。































 京公网安备 11010802027423号
京公网安备 11010802027423号