当前位置:
X-MOL 学术
›
Biomacromolecules
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Sericin Promotes Fibroin Silk I Stabilization Across a Phase-Separation
Biomacromolecules ( IF 5.5 ) Pub Date : 2017-06-30 00:00:00 , DOI: 10.1021/acs.biomac.7b00549 Hyo Won Kwak 1 , Ji Eun Ju 1 , Munju Shin 1 , Chris Holland 2 , Ki Hoon Lee 1, 3, 4
Biomacromolecules ( IF 5.5 ) Pub Date : 2017-06-30 00:00:00 , DOI: 10.1021/acs.biomac.7b00549 Hyo Won Kwak 1 , Ji Eun Ju 1 , Munju Shin 1 , Chris Holland 2 , Ki Hoon Lee 1, 3, 4
Affiliation
|
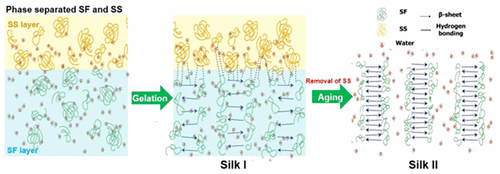
Natural silk spinning offers several advantages over the synthetic fiber spinning, although the underlying mechanisms of this process are yet to be fully elucidated. Silkworm silks, specifically B. mori, comprise two main proteins: fibroin, which forms the fiber, and sericin, a coextruded coating that acts as a matrix in the resulting nonwoven composite cocoon. To date, most studies have focused on fibroin’s self-assembly and gelation, with the influence of sericin during spinning receiving little to no attention. This study investigates sericin’s effects on the self-assembly of fibroin via their natural phase-separation. Through changes in sample opacity, FTIR, and XRD, we report that increasing sericin concentration retards the time to gelation and β-sheet formation of fibroin, causing it to adopt a Silk I conformation. Such findings have important implications for both the natural silk spinning process and any future industrial applications, suggesting that sericin may be able to induce long-range conformational and stability control in silk fibroin, while being in a separate phase, a factor that would facilitate long-term storage or silk feedstocks.
中文翻译:

丝胶促进跨相分离的丝蛋白I稳定化。
天然丝纺丝比合成纤维纺丝具有一些优势,尽管该过程的潜在机理尚待充分阐明。蚕丝,特别是桑蚕包含两种主要蛋白质:形成纤维的纤维蛋白和丝胶蛋白(一种共挤出的涂层,在所得的非织造复合茧中用作基质)。迄今为止,大多数研究都集中在纤维蛋白的自组装和凝胶化上,丝胶在纺丝过程中的影响几乎没有受到关注。这项研究调查了丝胶蛋白通过其自然相分离对丝蛋白的自组装的影响。通过改变样品的不透明度,FTIR和XRD,我们报告说,丝胶蛋白浓度的增加会延迟纤维蛋白的凝胶化和β片层形成的时间,从而导致其采用Silk I构象。这些发现对天然丝纺工艺和任何未来工业应用都具有重要意义,
更新日期:2017-07-02
中文翻译:

丝胶促进跨相分离的丝蛋白I稳定化。
天然丝纺丝比合成纤维纺丝具有一些优势,尽管该过程的潜在机理尚待充分阐明。蚕丝,特别是桑蚕包含两种主要蛋白质:形成纤维的纤维蛋白和丝胶蛋白(一种共挤出的涂层,在所得的非织造复合茧中用作基质)。迄今为止,大多数研究都集中在纤维蛋白的自组装和凝胶化上,丝胶在纺丝过程中的影响几乎没有受到关注。这项研究调查了丝胶蛋白通过其自然相分离对丝蛋白的自组装的影响。通过改变样品的不透明度,FTIR和XRD,我们报告说,丝胶蛋白浓度的增加会延迟纤维蛋白的凝胶化和β片层形成的时间,从而导致其采用Silk I构象。这些发现对天然丝纺工艺和任何未来工业应用都具有重要意义,











































 京公网安备 11010802027423号
京公网安备 11010802027423号