当前位置:
X-MOL 学术
›
Adv. Healthcare Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cotransporting Ion is a Trigger for Cellular Endocytosis of Transporter‐Targeting Nanoparticles: A Case Study of High‐Efficiency SLC22A5 (OCTN2)‐Mediated Carnitine‐Conjugated Nanoparticles for Oral Delivery of Therapeutic Drugs
Advanced Healthcare Materials ( IF 10.0 ) Pub Date : 2017-06-29 , DOI: 10.1002/adhm.201700165 Longfa Kou 1, 2 , Qing Yao 1 , Mengchi Sun 1 , Chunnuan Wu 3 , Jia Wang 1 , Qiuhua Luo 1 , Gang Wang 1 , Yuqian Du 1 , Qiang Fu 1 , Jian Wang 4 , Zhonggui He 5 , Vadivel Ganapathy 2 , Jin Sun 1
Advanced Healthcare Materials ( IF 10.0 ) Pub Date : 2017-06-29 , DOI: 10.1002/adhm.201700165 Longfa Kou 1, 2 , Qing Yao 1 , Mengchi Sun 1 , Chunnuan Wu 3 , Jia Wang 1 , Qiuhua Luo 1 , Gang Wang 1 , Yuqian Du 1 , Qiang Fu 1 , Jian Wang 4 , Zhonggui He 5 , Vadivel Ganapathy 2 , Jin Sun 1
Affiliation
|
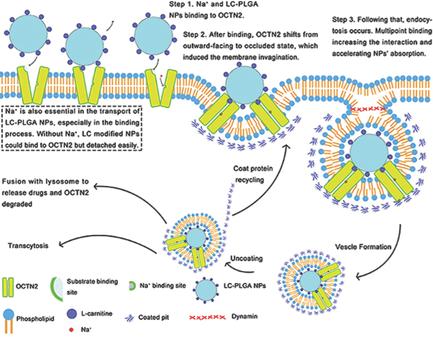
OCTN2 (SLC22A5) is a Na+‐coupled absorption transporter for l‐carnitine in small intestine. This study tests the potential of this transporter for oral delivery of therapeutic drugs encapsulated in l‐carnitine‐conjugated poly(lactic‐co‐glycolic acid) (PLGA) nanoparticles (LC‐PLGA NPs) and discloses the molecular mechanism for cellular endocytosis of transporter‐targeting nanoparticles. Conjugation of l‐carnitine to a surface of PLGA‐NPs enhances the cellular uptake and intestinal absorption of encapsulated drug. In both cases, the uptake process is dependent on cotransporting ion Na+. Computational OCTN2 docking analysis shows that the presence of Na+ is important for the formation of the energetically stable intermediate complex of transporter‐Na+‐LC‐PLGA NPs, which is also the first step in cellular endocytosis of nanoparticles. The transporter‐mediated intestinal absorption of LC‐PLGA NPs occurs via endocytosis/transcytosis rather than via the traditional transmembrane transport. The portal blood versus the lymphatic route is evaluated by the plasma appearance of the drug in the control and lymph duct‐ligated rats. Absorption via the lymphatic system is the predominant route in the oral delivery of the NPs. In summary, LC‐PLGA NPs can effectively target OCTN2 on the enterocytes for enhancing oral delivery of drugs and the critical role of cotransporting ions should be noticed in designing transporter‐targeting nanoparticles.
中文翻译:

共转运离子是针对转运蛋白的纳米颗粒的细胞内吞作用的触发因素:高效SLC22A5(OCTN2)介导的肉碱缀合纳米颗粒口服治疗药物的案例研究
OCTN2(SLC22A5)是中的Na + -偶联的吸收转运用于升在小肠肉碱。这项研究测试了这种转运蛋白口服包裹在l-肉碱共轭聚乳酸-乙醇酸(PLGA)纳米颗粒(LC-PLGA NPs)中的治疗药物的潜力,并揭示了转运蛋白细胞内吞的分子机制靶向纳米颗粒。缀合升肉碱到PLGA-NP的表面增强了细胞摄取和包封的药物的肠吸收。在这两种情况下,吸收过程均取决于共迁移离子Na +。计算OCTN2对接分析表明Na +的存在对于转运蛋白-Na + -LC-PLGA NPs的能量稳定的中间体复合物的形成非常重要,这也是纳米颗粒细胞内吞的第一步。LC‐PLGA NP的转运蛋白介导的肠道吸收是通过内吞/胞吞作用而不是通过传统的跨膜转运发生的。在对照和淋巴管结扎的大鼠中,通过药物的血浆外观评估门静脉血与淋巴途径之间的关系。经由淋巴系统的吸收是NPs口服递送的主要途径。总之,LC-PLGA NP可以有效地靶向肠上皮细胞上的OCTN2,以增强药物的口服递送,在设计靶向转运蛋白的纳米颗粒时应注意共转运离子的关键作用。
更新日期:2017-06-29
中文翻译:

共转运离子是针对转运蛋白的纳米颗粒的细胞内吞作用的触发因素:高效SLC22A5(OCTN2)介导的肉碱缀合纳米颗粒口服治疗药物的案例研究
OCTN2(SLC22A5)是中的Na + -偶联的吸收转运用于升在小肠肉碱。这项研究测试了这种转运蛋白口服包裹在l-肉碱共轭聚乳酸-乙醇酸(PLGA)纳米颗粒(LC-PLGA NPs)中的治疗药物的潜力,并揭示了转运蛋白细胞内吞的分子机制靶向纳米颗粒。缀合升肉碱到PLGA-NP的表面增强了细胞摄取和包封的药物的肠吸收。在这两种情况下,吸收过程均取决于共迁移离子Na +。计算OCTN2对接分析表明Na +的存在对于转运蛋白-Na + -LC-PLGA NPs的能量稳定的中间体复合物的形成非常重要,这也是纳米颗粒细胞内吞的第一步。LC‐PLGA NP的转运蛋白介导的肠道吸收是通过内吞/胞吞作用而不是通过传统的跨膜转运发生的。在对照和淋巴管结扎的大鼠中,通过药物的血浆外观评估门静脉血与淋巴途径之间的关系。经由淋巴系统的吸收是NPs口服递送的主要途径。总之,LC-PLGA NP可以有效地靶向肠上皮细胞上的OCTN2,以增强药物的口服递送,在设计靶向转运蛋白的纳米颗粒时应注意共转运离子的关键作用。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号