当前位置:
X-MOL 学术
›
Chem. Eur. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Diastereo‐ and Enantioselective Iridium Catalyzed Carbonyl (α‐Cyclopropyl)allylation via Transfer Hydrogenation
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2015-07-28 , DOI: 10.1002/chem.201502499
Ryosuke Tsutsumi , Suckchang Hong , Michael J. Krische
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2015-07-28 , DOI: 10.1002/chem.201502499
Ryosuke Tsutsumi , Suckchang Hong , Michael J. Krische
|
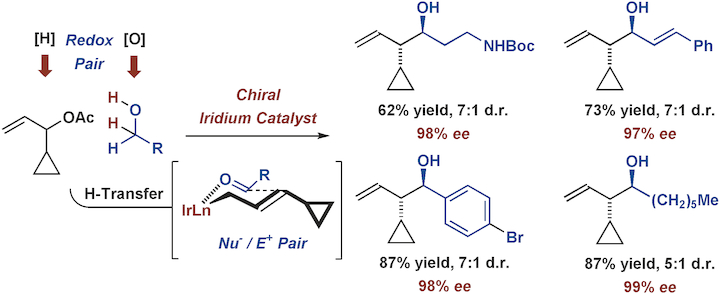
The first examples of diastereo‐ and enantioselective carbonyl α‐(cyclopropyl)allylation are reported. Under the conditions of iridium catalyzed transfer hydrogenation using the chiral precatalyst (R)‐Ir‐I modified by SEGPHOS, carbonyl α‐(cyclopropyl)allylation may be achieved with equal facility from alcohol or aldehyde oxidation levels. This methodology provides a conduit to hitherto inaccessible inaccessible enantiomerically enriched cyclopropane‐containing architectures.
中文翻译:

通过转移加氢催化非对映和对映选择性铱催化的羰基(α-环丙基)烯丙基化
报道了非对映和对映选择性羰基α-(环丙基)烯丙基化的第一个例子。在使用SEGPHOS改性的手性预催化剂(R)-Ir- I进行铱催化的转移加氢的条件下,醇或醛的氧化水平可以在相同的条件下实现羰基α-(环丙基)烯丙基化。这种方法学为迄今为止难以获得的对映异构体富集的含环丙烷的体系结构提供了一种途径。
更新日期:2015-07-28
中文翻译:

通过转移加氢催化非对映和对映选择性铱催化的羰基(α-环丙基)烯丙基化
报道了非对映和对映选择性羰基α-(环丙基)烯丙基化的第一个例子。在使用SEGPHOS改性的手性预催化剂(R)-Ir- I进行铱催化的转移加氢的条件下,醇或醛的氧化水平可以在相同的条件下实现羰基α-(环丙基)烯丙基化。这种方法学为迄今为止难以获得的对映异构体富集的含环丙烷的体系结构提供了一种途径。































 京公网安备 11010802027423号
京公网安备 11010802027423号