当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nickel‐Catalyzed Suzuki–Miyaura Coupling of a Tertiary Iodocyclopropane with Wide Boronic Acid Substrate Scope: Coupling Reaction Outcome Depends on Radical Species Stability
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2015-03-13 , DOI: 10.1002/adsc.201401000 Keisuke Yotsuji 1 , Naoyuki Hoshiya 1 , Takaaki Kobayashi 1 , Hayato Fukuda 1 , Hiroshi Abe 1 , Mitsuhiro Arisawa 1, 2 , Satoshi Shuto 1, 3
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2015-03-13 , DOI: 10.1002/adsc.201401000 Keisuke Yotsuji 1 , Naoyuki Hoshiya 1 , Takaaki Kobayashi 1 , Hayato Fukuda 1 , Hiroshi Abe 1 , Mitsuhiro Arisawa 1, 2 , Satoshi Shuto 1, 3
Affiliation
|
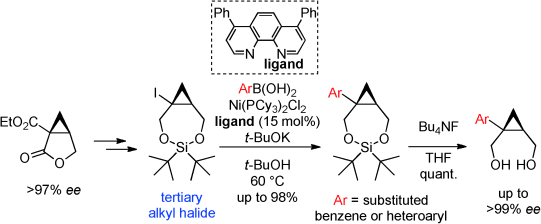
We describe a nickel‐catalyzed Suzuki–Miyaura arylation of a tertiary iodocyclopropane with arylboronic acids; this is an efficient and convergent strategy for providing various enantioenriched arylcyclopropanes with a quaternary stereogenic center. This is the first metal‐catalyzed coupling between a tertiary alkyl electrophile and a wide range of aromatics, including heteroaromatics. We found that the outcome of the Ni‐catalyzed coupling with halides as electrophiles was dependent on the stability of the radical species formed during the reaction. The use of tert‐butyl alcohol (t‐BuOH) as the reaction solvent was very effective, because of its stability under the radical‐generating reaction conditions.
中文翻译:

叔丁基碘代丙烷与宽硼酸基质的镍催化铃木-宫浦偶联作用范围:偶联反应结果取决于自由基的稳定性
我们描述了叔碘代环丙烷与芳基硼酸的镍催化的铃木-宫浦芳基化;这是为各种对映体富集的芳基环丙烷提供季立体形成中心的有效且收敛的策略。这是叔烷基亲电试剂与多种芳族化合物(包括杂芳族化合物)之间的首次金属催化偶联。我们发现,镍与卤化物作为亲电子试剂的偶合催化反应的结果取决于反应过程中形成的自由基种类的稳定性。使用叔丁醇(t- BuOH)作为反应溶剂非常有效,因为它在产生自由基的反应条件下具有稳定性。
更新日期:2015-03-13
中文翻译:

叔丁基碘代丙烷与宽硼酸基质的镍催化铃木-宫浦偶联作用范围:偶联反应结果取决于自由基的稳定性
我们描述了叔碘代环丙烷与芳基硼酸的镍催化的铃木-宫浦芳基化;这是为各种对映体富集的芳基环丙烷提供季立体形成中心的有效且收敛的策略。这是叔烷基亲电试剂与多种芳族化合物(包括杂芳族化合物)之间的首次金属催化偶联。我们发现,镍与卤化物作为亲电子试剂的偶合催化反应的结果取决于反应过程中形成的自由基种类的稳定性。使用叔丁醇(t- BuOH)作为反应溶剂非常有效,因为它在产生自由基的反应条件下具有稳定性。













































 京公网安备 11010802027423号
京公网安备 11010802027423号