当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Water Oxidation Kinetics of Accumulated Holes on the Surface of a TiO2 Photoanode: A Rate Law Analysis
ACS Catalysis ( IF 11.3 ) Pub Date : 2017-06-27 00:00:00 , DOI: 10.1021/acscatal.7b01150
Andreas Kafizas 1 , Yimeng Ma 1 , Ernest Pastor 1 , Stephanie R. Pendlebury 1 , Camilo Mesa 1 , Laia Francàs 1 , Florian Le Formal 2 , Nuruzzaman Noor 3 , Min Ling 3 , Carlos Sotelo-Vazquez 3 , Claire J. Carmalt 3 , Ivan P. Parkin 3 , James R. Durrant 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2017-06-27 00:00:00 , DOI: 10.1021/acscatal.7b01150
Andreas Kafizas 1 , Yimeng Ma 1 , Ernest Pastor 1 , Stephanie R. Pendlebury 1 , Camilo Mesa 1 , Laia Francàs 1 , Florian Le Formal 2 , Nuruzzaman Noor 3 , Min Ling 3 , Carlos Sotelo-Vazquez 3 , Claire J. Carmalt 3 , Ivan P. Parkin 3 , James R. Durrant 1
Affiliation
|
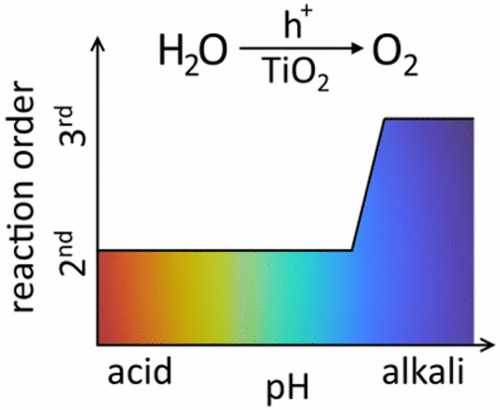
It has been more than 40 years since Fujishima and Honda demonstrated water splitting using TiO2, yet there is still no clear mechanism by which surface holes on TiO2 oxidize water. In this paper, we use a range of complementary techniques to study this reaction that provide a unique insight into the reaction mechanism. Using transient photocurrent and transient absorption spectroscopy, we measure both the kinetics of electron extraction (t50% ≈ 200 μs, 1.5VRHE) and the kinetics of hole oxidation of water (t50% ≈ 100 ms, 1.5VRHE) as a function of applied potential, demonstrating the water oxidation by TiO2 holes is the kinetic bottleneck in this water-splitting system. Photoinduced absorption spectroscopy measurements under 5 s LED irradiation are used to monitor the accumulation of surface TiO2 holes under conditions of photoelectrochemical water oxidation. Under these conditions, we find that the surface density of these holes increases nonlinearly with photocurrent density. In alkali (pH 13.6), this corresponded to a rate law for water oxidation that is third order with respect to surface hole density, with a rate constant kWO = 22 ± 2 nm4·s–1. Under neutral (pH = 6.7) and acidic (pH = 0.6) conditions, the rate law was second order with respect to surface hole density, indicative of a change in reaction mechanism. Although a change in reaction order was observed, the rate of reaction did not change significantly over the wide pH range examined (with TOFs per surface hole in the region of 20–25 s–1 at ∼1 sun irradiance). This showed that the rate-limiting step does not involve OH– nucleophilic attack and demonstrated the versatility of TiO2 as an active water oxidation photocatalyst over a wide range of pH.
中文翻译:

TiO 2光阳极表面累积空穴的水氧化动力学:速率规律分析
自藤岛和本田展示使用TiO 2进行水分解以来已经有40多年的历史了,但是仍然没有明确的机制可以使TiO 2上的表面孔氧化水。在本文中,我们使用了一系列辅助技术来研究该反应,从而提供了对反应机理的独特见解。使用瞬态光电流和瞬态吸收光谱法,我们测量了电子萃取的动力学(t 50% ≈200μs,1.5 V RHE)和水的空穴氧化动力学(t 50% ≈100 ms,1.5 V RHE)。电位的作用,证明水被TiO 2氧化孔是此水分解系统中的动力学瓶颈。在5 s LED照射下的光致吸收光谱测量用于监测在光电化学水氧化条件下表面TiO 2孔的积累。在这些条件下,我们发现这些孔的表面密度随光电流密度非线性增加。在碱(pH 13.6)中,这对应于水氧化的速率定律,该定律相对于表面空穴密度是三阶的,速率常数k WO = 22±2 nm 4 ·s –1。在中性(pH = 6.7)和酸性(pH = 0.6)条件下,速率法则相对于表面空穴密度是二阶的,表明反应机理发生了变化。尽管观察到反应顺序发生了变化,但在较宽的pH值范围内(在约1个太阳辐射下,每个表面孔的TOF在20-25 s - 1范围内),反应速率均没有显着变化。这表明限速步骤不涉及OH –亲核攻击,并证明了TiO 2作为活性水氧化光催化剂在广泛的pH范围内的多功能性。
更新日期:2017-06-28
中文翻译:

TiO 2光阳极表面累积空穴的水氧化动力学:速率规律分析
自藤岛和本田展示使用TiO 2进行水分解以来已经有40多年的历史了,但是仍然没有明确的机制可以使TiO 2上的表面孔氧化水。在本文中,我们使用了一系列辅助技术来研究该反应,从而提供了对反应机理的独特见解。使用瞬态光电流和瞬态吸收光谱法,我们测量了电子萃取的动力学(t 50% ≈200μs,1.5 V RHE)和水的空穴氧化动力学(t 50% ≈100 ms,1.5 V RHE)。电位的作用,证明水被TiO 2氧化孔是此水分解系统中的动力学瓶颈。在5 s LED照射下的光致吸收光谱测量用于监测在光电化学水氧化条件下表面TiO 2孔的积累。在这些条件下,我们发现这些孔的表面密度随光电流密度非线性增加。在碱(pH 13.6)中,这对应于水氧化的速率定律,该定律相对于表面空穴密度是三阶的,速率常数k WO = 22±2 nm 4 ·s –1。在中性(pH = 6.7)和酸性(pH = 0.6)条件下,速率法则相对于表面空穴密度是二阶的,表明反应机理发生了变化。尽管观察到反应顺序发生了变化,但在较宽的pH值范围内(在约1个太阳辐射下,每个表面孔的TOF在20-25 s - 1范围内),反应速率均没有显着变化。这表明限速步骤不涉及OH –亲核攻击,并证明了TiO 2作为活性水氧化光催化剂在广泛的pH范围内的多功能性。































 京公网安备 11010802027423号
京公网安备 11010802027423号