当前位置:
X-MOL 学术
›
Nat. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A practical and catalyst-free trifluoroethylation reaction of amines using trifluoroacetic acid.
Nature Communications ( IF 14.7 ) Pub Date : 2017-06-26 , DOI: 10.1038/ncomms15913
Keith G. Andrews , Radmila Faizova , Ross M. Denton
Nature Communications ( IF 14.7 ) Pub Date : 2017-06-26 , DOI: 10.1038/ncomms15913
Keith G. Andrews , Radmila Faizova , Ross M. Denton
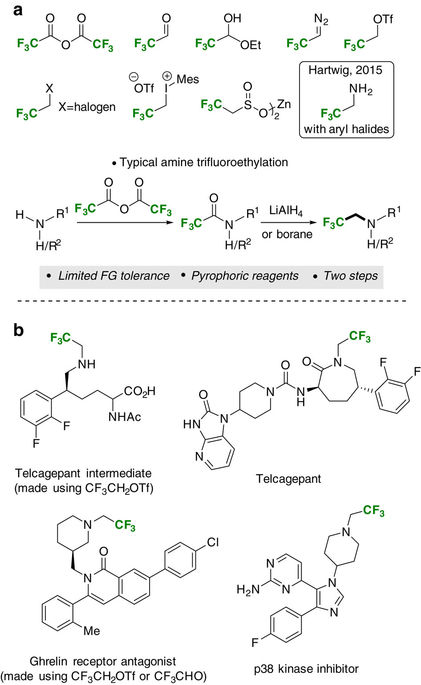
|
Amines are a fundamentally important class of biologically active compounds and the ability to manipulate their physicochemical properties through the introduction of fluorine is of paramount importance in medicinal chemistry. Current synthesis methods for the construction of fluorinated amines rely on air and moisture sensitive reagents that require special handling or harsh reductants that limit functionality. Here we report practical, catalyst-free, reductive trifluoroethylation reactions of free amines exhibiting remarkable functional group tolerance. The reactions proceed in conventional glassware without rigorous exclusion of either moisture or oxygen, and use trifluoroacetic acid as a stable and inexpensive fluorine source. The new methods provide access to a wide range of medicinally relevant functionalized tertiary β-fluoroalkylamine cores, either through direct trifluoroethylation of secondary amines or via a three-component coupling of primary amines, aldehydes and trifluoroacetic acid. A reduction of in situ-generated silyl ester species is proposed to account for the reductive selectivity observed.
中文翻译:

使用三氟乙酸进行的实用且无催化剂的胺三氟乙基化反应。
胺是一类重要的生物活性化合物,通过引入氟来控制其理化性质的能力在药物化学中至关重要。当前用于构造氟化胺的合成方法依赖于对空气和湿气敏感的试剂,这些试剂需要特殊的处理或苛刻的还原剂,从而限制了其功能性。在这里我们报告了表现出显着的官能团耐受性的游离胺的实用,无催化剂的还原性三氟乙基化反应。反应在不严格排除水分或氧气的情况下在常规玻璃器皿中进行,并使用三氟乙酸作为稳定且廉价的氟源。通过仲胺的直接三氟乙基化或伯胺,醛和三氟乙酸的三组分偶联,新方法提供了广泛的与医学相关的官能化叔丁基氟烷基胺核的使用途径。提出减少原位产生的甲硅烷基酯种类以解决所观察到的还原选择性。
更新日期:2017-06-27
中文翻译:

使用三氟乙酸进行的实用且无催化剂的胺三氟乙基化反应。
胺是一类重要的生物活性化合物,通过引入氟来控制其理化性质的能力在药物化学中至关重要。当前用于构造氟化胺的合成方法依赖于对空气和湿气敏感的试剂,这些试剂需要特殊的处理或苛刻的还原剂,从而限制了其功能性。在这里我们报告了表现出显着的官能团耐受性的游离胺的实用,无催化剂的还原性三氟乙基化反应。反应在不严格排除水分或氧气的情况下在常规玻璃器皿中进行,并使用三氟乙酸作为稳定且廉价的氟源。通过仲胺的直接三氟乙基化或伯胺,醛和三氟乙酸的三组分偶联,新方法提供了广泛的与医学相关的官能化叔丁基氟烷基胺核的使用途径。提出减少原位产生的甲硅烷基酯种类以解决所观察到的还原选择性。

































 京公网安备 11010802027423号
京公网安备 11010802027423号