Polymer ( IF 4.1 ) Pub Date : 2017-06-16 , DOI: 10.1016/j.polymer.2017.06.029 Hongjun Yang , Aibin Sun , Chenqun Chai , Wenyan Huang , Xiaoqiang Xue , Jianhai Chen , Bibiao Jiang
|
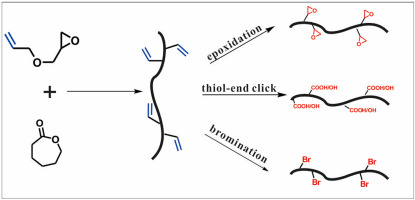
Aliphatic polyesters have been widely used in environmental and biomedical engineering, but a lack of functional groups limits their applications. Here, we reported a facile approach to synthesize vinyl functional polyester via the ring opening copolymerization of ε-caprolactone (CL) and allyl glycidyl ether (AGE). NMR analysis confirmed the copolymeric structures and suggested that the copolymerization depended on the epoxide ring of AGE rather than vinyl group. The amount of AGE incorporated into the copolymers (FAGE) increased with the amount of epoxide monomer feed with a maximum incorporation of 16.7%. Increasing temperature helped AGE to incorporate into the copolymer, however, accompanying with lots amount of AGE homopolymers. The resulting copolymer was successfully post-functionalized by thiol-end click, epoxidation, and bromination reactions depending on the reactivity of pendent ally groups. This facile and efficient approach can be used to functionalize biodegradable polymers and synthesize some new polymers under mild conditions.
中文翻译:

含烯丙基侧基的可降解脂族聚酯的合成和后官能化
脂肪族聚酯已广泛用于环境和生物医学工程中,但是缺乏官能团限制了它们的应用。在这里,我们报道了一种通过ε-己内酯(CL)和烯丙基缩水甘油醚(AGE)的开环共聚来合成乙烯基官能聚酯的简便方法。NMR分析证实了共聚物的结构,并表明共聚取决于AGE的环而不是乙烯基。掺入共聚物中的AGE量(F AGE)随环氧单体进料量的增加而增加,最大掺入量为16.7%。温度的升高有助于AGE掺入共聚物中,但是伴随着大量的AGE均聚物。取决于共聚物侧基的反应性,通过硫醇末端点击,环氧化和溴化反应成功地将所得共聚物后官能化。这种简便有效的方法可用于使可生物降解的聚合物功能化,并在温和的条件下合成一些新的聚合物。

































 京公网安备 11010802027423号
京公网安备 11010802027423号