当前位置:
X-MOL 学术
›
Tetrahedron
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reaction of 1-bromonaphthalene with PH3 in the t-BuOK/DMSO system: PCl3-free synthesis of di(1-naphthyl)phosphine and its oxide
Tetrahedron ( IF 2.1 ) Pub Date : 2017-06-23 , DOI: 10.1016/j.tet.2017.06.036 Vladimir A. Kuimov , Elena A. Matveeva , Spartak S. Khutsishvili , Tamara I. Vakul'skaya , Lidiya M. Sinegovskaya , Svetlana F. Malysheva , Nina K. Gusarova , Boris A. Trofimov
中文翻译:

1-溴萘与PH 3在t -BuOK / DMSO系统中的反应:无PCl 3的二(1-萘基)膦及其氧化物的合成
更新日期:2017-06-23
Tetrahedron ( IF 2.1 ) Pub Date : 2017-06-23 , DOI: 10.1016/j.tet.2017.06.036 Vladimir A. Kuimov , Elena A. Matveeva , Spartak S. Khutsishvili , Tamara I. Vakul'skaya , Lidiya M. Sinegovskaya , Svetlana F. Malysheva , Nina K. Gusarova , Boris A. Trofimov
|
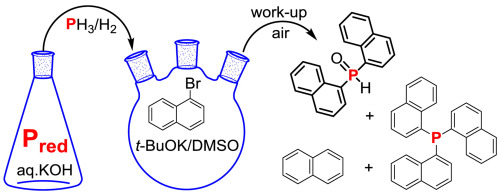
The phosphine, generated together with hydrogen from red phosphorus and aqueous KOH, reacts with 1-bromonaphthalene in the t-BuOK/DMSO system under mild conditions (70 °C, atmospheric pressure) to give di(1-naphthyl)phosphine, which is easily oxidized in the presence of air to afford di(1-naphthyl)phosphine oxide in 45% preparative yield. Tri(1-naphthyl)phosphine and naphthalene are also formed in the reaction in 23 and 27% yield, respectively. According to ESR and UV data, the studied phosphination of 1-bromonaphthalene involves a single electron transfer process.
中文翻译:

1-溴萘与PH 3在t -BuOK / DMSO系统中的反应:无PCl 3的二(1-萘基)膦及其氧化物的合成
膦,用氢气与在1-溴萘一起产生从红磷和KOH水溶液,进行反应吨温和的条件(70℃,大气压),得到二(1-萘基)膦,其是下-BuOK / DMSO体系在空气中易氧化,以45%的制备产率得到二(1-萘基)氧化膦。在反应中也分别形成三(1-萘基)膦和萘,产率为23%和27%。根据ESR和UV数据,所研究的1-溴萘的磷酸化涉及单个电子转移过程。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号