Applied Catalysis B: Environment and Energy ( IF 20.2 ) Pub Date : 2017-06-19 , DOI: 10.1016/j.apcatb.2017.06.045 Batsile M. Mogudi , Phendukani Ncube , Ndzondelelo Bingwa , Naphtaly Mawila , Shitshembiso Mathebula , Reinout Meijboom
|
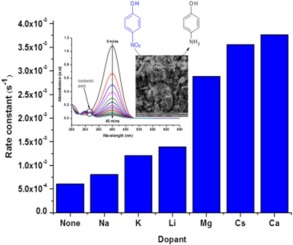
Mesoporous cobalt oxides doped with alkali (Li, Na, K, Cs) and alkaline earth (Mg, Ca) metals were synthesized and evaluated for their catalytic activity in the reduction of 4-nitrophenol. The prepared materials were characterized using scanning electron microscopy (SEM), transmission electron microscopy (TEM), powder X-ray diffraction (XRD), Brunauer–Emmet–Teller (BET) and hydrogen temperature programmed reduction (H2-TPR) analyses. The characterization techniques used showed the materials to consist of mono-dispersed nanoparticle aggregates with connected, well defined intra-particle voids and the crystalline phase of the cobalt oxide to be cubic Co3O4, while the pore diameters ranged from 12.1 to 19.2 nm depending on the metal ion dopant. The reduction of 4-nitrophenol was chosen as a well-controlled model reaction allowing us to determine the catalytic activity as a function of alkali or alkaline earth dopant. Calcium doped cobalt oxide was found to be the most catalytically active with an apparent rate constant of 3.76 × 10−3 s−1 and the order with respect to dopant was Ca > Cs > Mg > Li > K > Na. From characterization of the catalysts by SEM, TEM and H2-TPR promotion effects of the dopants were found to be due to electronic changes in the catalysts as a result of doping rather than structural changes. The kinetics of the most active calcium doped catalyst was modeled in terms of Langmuir–Hinshelwood kinetics. The Langmuir–Hinshelwood surface rate constant for Ca-doped Co3O4 was 5.47 × 10−5 mol m−2 s−1 compared to the undoped Co3O4 at 5.33 × 10−6 mol m−2 s−1. Activation energies were calculated to be 51.3 kJ mol−1 and 50.7 kJ mol−1 for undoped and Ca-doped Co3O4.
中文翻译:

碱金属和碱土金属对介孔Co 3 O 4催化4-硝基苯酚还原的催化作用
合成了掺杂有碱金属(Li,Na,K,Cs)和碱土金属(Mg,Ca)的中孔氧化钴,并评估了它们在还原4-硝基苯酚中的催化活性。使用扫描电子显微镜(SEM),透射电子显微镜(TEM),粉末X射线衍射(XRD),Brunauer–Emmet–Teller(BET)和氢程序升温还原(H 2 -TPR)分析对制备的材料进行表征。所用的表征技术表明,该材料由单分散的纳米颗粒聚集体组成,这些聚集体具有连通的,定义明确的颗粒内空隙,并且氧化钴的结晶相为立方晶的Co 3 O 4。,而孔径根据金属离子掺杂剂在12.1至19.2nm范围内。选择4-硝基苯酚的还原反应是一个控制良好的模型反应,使我们能够确定作为碱或碱土金属掺杂剂的函数的催化活性。发现掺杂钙的氧化钴最具催化活性,其表观速率常数为3.76×10 -3 s -1,并且相对于掺杂剂的顺序为Ca> Cs> Mg> Li> K> Na。由SEM,TEM和H 2表征催化剂发现掺杂剂的TPR促进作用是由于掺杂而不是结构变化导致催化剂中的电子变化。用Langmuir-Hinshelwood动力学模拟了活性最高的钙掺杂催化剂的动力学。对于CA掺杂钴的朗缪尔-欣谢尔伍德表面速率常数3 ö 4为5.47×10 -5 摩尔米-2 小号-1相比未掺杂共3 ö 4在5.33×10 -6 摩尔米-2 小号-1。计算出未掺杂和掺杂Ca的Co 3的活化能为51.3 kJ mol -1和50.7 kJ mol -1O 4。































 京公网安备 11010802027423号
京公网安备 11010802027423号