当前位置:
X-MOL 学术
›
Nat. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A bioinspired and biocompatible ortho-sulfiliminyl phenol synthesis.
Nature Communications ( IF 14.7 ) Pub Date : 2017-06-19 , DOI: 10.1038/ncomms15912 Feng Xiong , Liang Lu , Tian-Yu Sun , Qian Wu , Dingyuan Yan , Ying Chen , Xinhao Zhang , Wei Wei , Yi Lu , Wei-Yin Sun , Jie Jack Li , Jing Zhao
Nature Communications ( IF 14.7 ) Pub Date : 2017-06-19 , DOI: 10.1038/ncomms15912 Feng Xiong , Liang Lu , Tian-Yu Sun , Qian Wu , Dingyuan Yan , Ying Chen , Xinhao Zhang , Wei Wei , Yi Lu , Wei-Yin Sun , Jie Jack Li , Jing Zhao
|
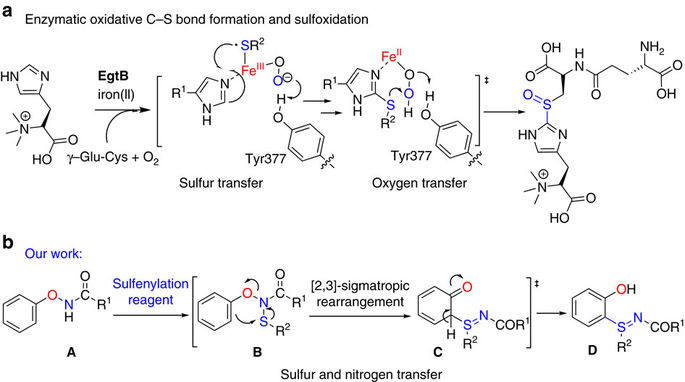
Synthetic methods inspired by Nature often offer unique advantages including mild conditions and biocompatibility with aqueous media. Inspired by an ergothioneine biosynthesis protein EgtB, a mononuclear non-haem iron enzyme capable of catalysing the C-S bond formation and sulfoxidation, herein, we discovered a mild and metal-free C-H sulfenylation/intramolecular rearrangement cascade reaction employing an internally oxidizing O-N bond as a directing group. Our strategy accommodates a variety of oxyamines with good site selectivity and intrinsic oxidative properties. Combining an O-N bond with an X-S bond generates a C-S bond and an S=N bond rapidly. The newly discovered cascade reaction showed excellent chemoselectivity and a wide substrate scope for both oxyamines and sulfenylation reagents. We demonstrated the biocompatibility of the C-S bond coupling reaction by applying a coumarin-based fluorogenic probe in bacterial lysates. Finally, the C-S bond coupling reaction enabled the first fluorogenic formation of phospholipids, which self-assembled to fluorescent vesicles in situ.
中文翻译:

具有生物启发性和生物相容性的邻磺酰亚胺基苯酚合成。
受自然启发的合成方法通常具有独特的优势,包括温和的条件以及与水性介质的生物相容性。受到麦角硫氨酸生物合成蛋白EgtB(一种能够催化CS键形成和亚砜氧化的单核非血红素铁酶)的启发,在本文中,我们发现了一种温和且无金属的CH磺酰化/分子内重排级联反应,该反应使用内部氧化的ON键作为指导小组。我们的策略可容纳各种具有良好的位点选择性和固有氧化性能的氧胺。将ON键与XS键结合可快速生成CS键和S = N键。新发现的级联反应显示出优异的化学选择性和广泛的氧胺和亚磺酰化试剂的底物范围。我们通过在细菌裂解物中应用基于香豆素的荧光探针证明了CS键偶联反应的生物相容性。最后,CS键偶联反应使磷脂的第一荧光形成成为可能,所述磷脂自发地自组装成荧光囊泡。
更新日期:2017-06-26
中文翻译:

具有生物启发性和生物相容性的邻磺酰亚胺基苯酚合成。
受自然启发的合成方法通常具有独特的优势,包括温和的条件以及与水性介质的生物相容性。受到麦角硫氨酸生物合成蛋白EgtB(一种能够催化CS键形成和亚砜氧化的单核非血红素铁酶)的启发,在本文中,我们发现了一种温和且无金属的CH磺酰化/分子内重排级联反应,该反应使用内部氧化的ON键作为指导小组。我们的策略可容纳各种具有良好的位点选择性和固有氧化性能的氧胺。将ON键与XS键结合可快速生成CS键和S = N键。新发现的级联反应显示出优异的化学选择性和广泛的氧胺和亚磺酰化试剂的底物范围。我们通过在细菌裂解物中应用基于香豆素的荧光探针证明了CS键偶联反应的生物相容性。最后,CS键偶联反应使磷脂的第一荧光形成成为可能,所述磷脂自发地自组装成荧光囊泡。































 京公网安备 11010802027423号
京公网安备 11010802027423号