当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Trimethylenemethane Diyl Mediated Tandem Cycloaddition Reactions: Mechanism Based Design of Synthetic Strategies
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2015-07-23 00:00:00 , DOI: 10.1021/acs.accounts.5b00178
Hee-Yoon Lee 1
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2015-07-23 00:00:00 , DOI: 10.1021/acs.accounts.5b00178
Hee-Yoon Lee 1
Affiliation
|
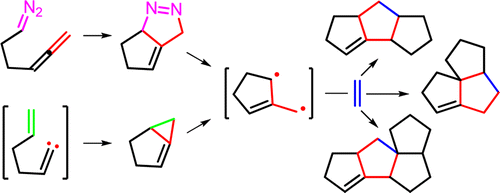
Several criteria for the measure of synthetic strategies toward “ideal synthesis” are available to guide the design and evaluation of the synthetic strategies toward the target molecules. One strategy toward “ideal synthesis” is developing a multistep reaction that involves dramatic change in complexity. Biogenesis of natural products and mechanistic investigation of complicated organic transformation provide good inspiration for design of new synthetic strategies. Trimethylenemethane diradical (TMM diyl), first introduced only as a theoretically interesting structure 60 years ago, gained interests of physical organic chemistry when it was first detected by Dowd. Study of characteristics and properties of TMM diyl was accelerated in a great deal when Koebrich observed dimeric hydrocarbon products from the reaction of 1,1-dibromo-2-methylhexa-1,5-diene with MeLi. Berson followed the mechanistic investigation of the reaction that involved 2-methylenecyclopentane-1,3-diyl, and thoroughly studied physical and chemical properties of the TMM diyl. This lead to the development of intramolecular [2 + 3] TMM diyl cycloaddition reaction for the construction of linearly fused triquinanes by Little.
中文翻译:

三亚甲基二甲酰基介导的串联环加成反应:基于机理的合成策略设计
可以使用几种衡量“理想合成”合成策略的标准来指导针对目标分子的合成策略的设计和评估。迈向“理想合成”的一种策略是开发一种涉及复杂性急剧变化的多步反应。天然产物的生物发生和复杂有机转化的机理研究为设计新的合成策略提供了很好的启示。三亚甲基二自由基(TMM diyl),仅在60年前作为一种理论上令人感兴趣的结构而引入,当它被Dowd首次发现时,引起了人们对物理有机化学的兴趣。当Koebrich观察到1,1-二溴-2-甲基六-1的反应生成的二聚烃产物时,大大加快了TMM二基特性和性质的研究。5-二烯与MeLi。Berson跟踪了涉及2-亚甲基环戊烷-1,3-二基的反应机理,并彻底研究了TMM二基的物理和化学性质。这导致了Little的分子内[2 + 3] TMM二基环加成反应的发展,用于构建线性稠合的三喹烷。
更新日期:2015-07-23
中文翻译:

三亚甲基二甲酰基介导的串联环加成反应:基于机理的合成策略设计
可以使用几种衡量“理想合成”合成策略的标准来指导针对目标分子的合成策略的设计和评估。迈向“理想合成”的一种策略是开发一种涉及复杂性急剧变化的多步反应。天然产物的生物发生和复杂有机转化的机理研究为设计新的合成策略提供了很好的启示。三亚甲基二自由基(TMM diyl),仅在60年前作为一种理论上令人感兴趣的结构而引入,当它被Dowd首次发现时,引起了人们对物理有机化学的兴趣。当Koebrich观察到1,1-二溴-2-甲基六-1的反应生成的二聚烃产物时,大大加快了TMM二基特性和性质的研究。5-二烯与MeLi。Berson跟踪了涉及2-亚甲基环戊烷-1,3-二基的反应机理,并彻底研究了TMM二基的物理和化学性质。这导致了Little的分子内[2 + 3] TMM二基环加成反应的发展,用于构建线性稠合的三喹烷。































 京公网安备 11010802027423号
京公网安备 11010802027423号