Tetrahedron ( IF 2.1 ) Pub Date : 2017-06-16 , DOI: 10.1016/j.tet.2017.06.023 Richard C. Knighton , Adrian B. Chaplin
|
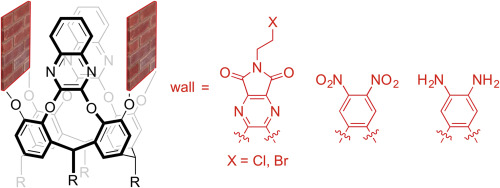
The synthesis of four new dissymmetric cavitands is reported. These deep-walled receptors are constructed from a resorcin[4]arene scaffold bearing anti-disposed quinoxaline substituents, with either N-haloalkyl-diazaphthalimide (1), dinitrophenyl (2) or diaminophenyl (3) moieties as the other wall components. The structure and inclusion properties of 1 and 2 have been probed in solution by NMR spectroscopy and notably in the solid-state by X-ray crystallography. The diazaphthalimide-based compounds 1 crystallise as 1:1 host-guest complexes with chloroform, with the resorcin[4]arene scaffolds adopting pinched cone conformations. Conversely, the dinitrophenyl-variant 2 features a more open, symmetric structure in the solid-state and co-crystallises with two acetone molecules within the central cavity. Preliminary binding experiments in mesitylene-d12 at 303 K demonstrate 1 (Kapp = 5 × 102 M−1) and 2 (Kapp = 2 × 102 M−1) are effective hosts for cyclohexane guest molecules in the absence of competitive solvent inclusion.
中文翻译:

一系列不对称间苯二酚[4]芳烃基空洞分子的合成,结构和结合性能
据报道,合成了四个新的不对称空泡体。这些深壁受体由带有抗位错喹喔啉取代基的间苯二酚[4]芳烃骨架构成,N-卤代烷基-二氮杂苯二甲酰亚胺(1),二硝基苯基(2)或二氨基苯基(3)部分为其他壁成分。1和2的结构和内含物性质已通过NMR光谱在溶液中进行了探测,特别是通过X射线晶体学在固态中进行了探测。二氮杂苯二甲酰亚胺基化合物1与氯仿结晶为1:1主宾复合物,间苯二酚[4]芳烃骨架采用捏合的圆锥构象。相反,二硝基苯基变体2在固态时具有更开放的对称结构,并与中心腔内的两个丙酮分子共结晶。 在303 K的均三甲苯基d 12中的初步结合实验证明1(K app = 5×10 2 M -1)和2(K app = 2×10 2 M -1)是环己烷客体分子的有效主体。竞争性溶剂包合。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号