当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structure Based Design of N-(3-((1H-Pyrazolo[3,4-b]pyridin-5-yl)ethynyl)benzenesulfonamides as Selective Leucine-Zipper and Sterile-α Motif Kinase (ZAK) Inhibitors
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2017-06-16 00:00:00 , DOI: 10.1021/acs.jmedchem.7b00572 Yu Chang,Xiaoyun Lu,Marthandam Asokan Shibu,Yi-Bo Dai,Jinfeng Luo,Yan Zhang,Yingjun Li,Peng Zhao,Zhang Zhang,Yong Xu,Zheng-Chao Tu,Qing-Wen Zhang,Cai-Hong Yun,Chih-Yang Huang,Ke Ding
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2017-06-16 00:00:00 , DOI: 10.1021/acs.jmedchem.7b00572 Yu Chang,Xiaoyun Lu,Marthandam Asokan Shibu,Yi-Bo Dai,Jinfeng Luo,Yan Zhang,Yingjun Li,Peng Zhao,Zhang Zhang,Yong Xu,Zheng-Chao Tu,Qing-Wen Zhang,Cai-Hong Yun,Chih-Yang Huang,Ke Ding
|
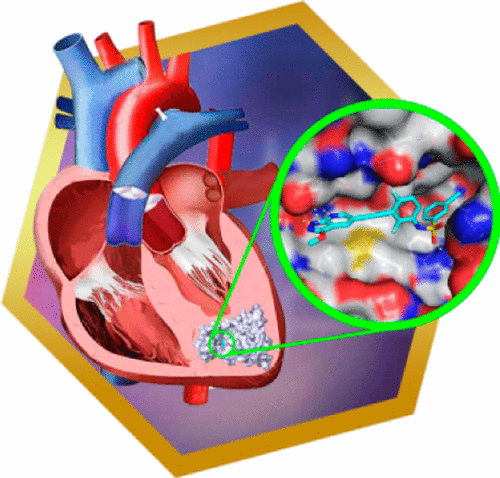
A series of N-(3-((1H-pyrazolo[3,4-b]pyridin-5-yl)ethynyl)benzenesulfonamides were designed as the first class of highly selective ZAK inhibitors. The representative compound 3h strongly inhibits the kinase activity of ZAK with an IC50 of 3.3 nM and dose-dependently suppresses the activation of ZAK downstream signals in vitro and in vivo, while it is significantly less potent for the majority of 403 nonmutated kinases evaluated. Compound 3h also exhibits orally therapeutic effects on cardiac hypertrophy in a spontaneous hypertensive rat model.
中文翻译:

的结构设计基于ñ - (3 - ((1 ħ -吡唑并[3,4- b ]吡啶-5-基)乙炔基)苯磺酰胺作为选择性亮氨酸拉链和无菌-α基序激酶(ZAK)抑制剂
设计了一系列N-(3-((1 H-吡唑并[3,4- b ]吡啶基-5-基)乙炔基)苯磺酰胺作为第一类高选择性ZAK抑制剂。代表性化合物3h强烈抑制该激酶ZAK的活性为IC 50为3.3 nM,并在体内和体外剂量依赖性地抑制ZAK下游信号的激活,而对大多数403种未突变激酶的效价则明显较低,化合物3h还在以下方面表现出口服治疗作用:自发性高血压大鼠模型中的心脏肥大。
更新日期:2017-06-28
中文翻译:

的结构设计基于ñ - (3 - ((1 ħ -吡唑并[3,4- b ]吡啶-5-基)乙炔基)苯磺酰胺作为选择性亮氨酸拉链和无菌-α基序激酶(ZAK)抑制剂
设计了一系列N-(3-((1 H-吡唑并[3,4- b ]吡啶基-5-基)乙炔基)苯磺酰胺作为第一类高选择性ZAK抑制剂。代表性化合物3h强烈抑制该激酶ZAK的活性为IC 50为3.3 nM,并在体内和体外剂量依赖性地抑制ZAK下游信号的激活,而对大多数403种未突变激酶的效价则明显较低,化合物3h还在以下方面表现出口服治疗作用:自发性高血压大鼠模型中的心脏肥大。






























 京公网安备 11010802027423号
京公网安备 11010802027423号