当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Catalytic Enantioselective Aza‐pinacol Rearrangement
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2017-06-29 , DOI: 10.1002/anie.201705539
Yuanyuan Yu 1 , Jingwen Li 2 , Long Jiang 1 , Jing-Ren Zhang 2, 3 , Liansuo Zu 1, 3
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2017-06-29 , DOI: 10.1002/anie.201705539
Yuanyuan Yu 1 , Jingwen Li 2 , Long Jiang 1 , Jing-Ren Zhang 2, 3 , Liansuo Zu 1, 3
Affiliation
|
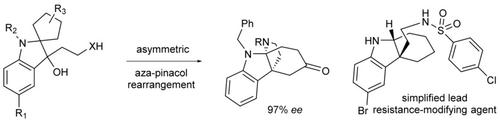
The first catalytic enantioselective asymmetric aza‐pinacol rearrangement is reported. The reactions are catalyzed by a chiral phosphoric acid and proceed via a highly organized transition state involving a cyclic aza‐ortho‐xylylene intermediate to afford the indoline structures with good to excellent enantioselectivity. The synthetic utility of this method is demonstrated by the asymmetric synthesis of a key intermediate to the natural product minfiensine and the identification of a chiral lead compound to repress antibiotic resistance.
中文翻译:

催化对映选择性氮杂频哪醇重排
报道了第一个催化对映选择性不对称氮杂频哪醇重排。该反应由手性磷酸催化,并通过高度结构化的过渡态进行,该过渡态涉及环状氮杂邻二甲苯基中间体,从而提供具有良好至优异对映选择性的二氢吲哚结构。该方法的合成效用通过天然产物米非芬辛的关键中间体的不对称合成以及鉴定出可抑制抗生素耐药性的手性前导化合物而得到证明。
更新日期:2017-06-29
中文翻译:

催化对映选择性氮杂频哪醇重排
报道了第一个催化对映选择性不对称氮杂频哪醇重排。该反应由手性磷酸催化,并通过高度结构化的过渡态进行,该过渡态涉及环状氮杂邻二甲苯基中间体,从而提供具有良好至优异对映选择性的二氢吲哚结构。该方法的合成效用通过天然产物米非芬辛的关键中间体的不对称合成以及鉴定出可抑制抗生素耐药性的手性前导化合物而得到证明。

































 京公网安备 11010802027423号
京公网安备 11010802027423号