当前位置:
X-MOL 学术
›
Nat. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cryptic indole hydroxylation by a non-canonical terpenoid cyclase parallels bacterial xenobiotic detoxification.
Nature Communications ( IF 14.7 ) Pub Date : 2017-06-15 , DOI: 10.1038/ncomms15804 Susann Kugel , Martin Baunach , Philipp Baer , Mie Ishida-Ito , Srividhya Sundaram , Zhongli Xu , Michael Groll , Christian Hertweck
Nature Communications ( IF 14.7 ) Pub Date : 2017-06-15 , DOI: 10.1038/ncomms15804 Susann Kugel , Martin Baunach , Philipp Baer , Mie Ishida-Ito , Srividhya Sundaram , Zhongli Xu , Michael Groll , Christian Hertweck
|
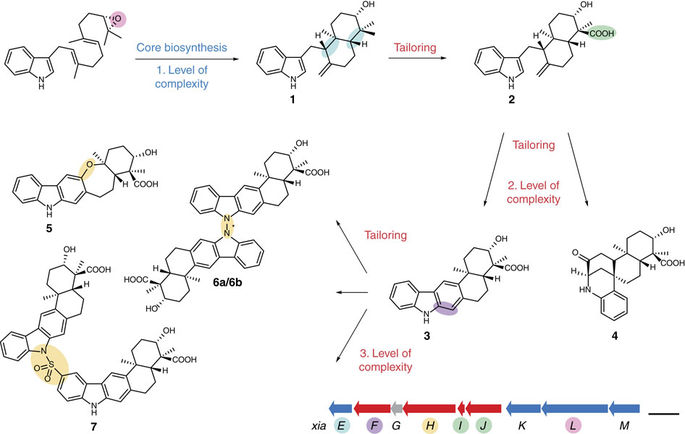
Terpenoid natural products comprise a wide range of molecular architectures that typically result from C-C bond formations catalysed by classical type I/II terpene cyclases. However, the molecular diversity of biologically active terpenoids is substantially increased by fully unrelated, non-canonical terpenoid cyclases. Their evolutionary origin has remained enigmatic. Here we report the in vitro reconstitution of an unusual flavin-dependent bacterial indoloterpenoid cyclase, XiaF, together with a designated flavoenzyme-reductase (XiaP) that mediates a key step in xiamycin biosynthesis. The crystal structure of XiaF with bound FADH2 (at 2.4 Å resolution) and phylogenetic analyses reveal that XiaF is, surprisingly, most closely related to xenobiotic-degrading enzymes. Biotransformation assays show that XiaF is a designated indole hydroxylase that can be used for the production of indigo and indirubin. We unveil a cryptic hydroxylation step that sets the basis for terpenoid cyclization and suggest that the cyclase has evolved from xenobiotics detoxification enzymes.
中文翻译:

通过非规范的萜类环化酶进行的吲哚羟化作用与细菌异源生物的解毒作用平行。
萜类天然产物包含多种分子结构,通常由经典I / II型萜烯环化酶催化的CC键形成所致。然而,完全不相关的,非规范的萜类环化酶大大提高了生物活性萜类的分子多样性。它们的进化起源一直是谜。在这里,我们报告了不寻常的黄素依赖性细菌吲哚萜类化合物环化酶XiaF,以及指定的黄素酶还原酶(XiaP)的体外重组,该酶介导了烟霉素生物合成的关键步骤。结合FADH 2的XiaF的晶体结构(在2.4Å分辨率下)和系统发育分析表明,XiaF与异源降解酶最密切相关。生物转化试验表明,XiaF是一种指定的吲哚羟化酶,可用于生产靛蓝和靛玉红。我们揭示了一种隐蔽的羟基化步骤,该步骤为萜类化合物环化奠定了基础,并表明环化酶已从异源生物解毒酶演变而来。
更新日期:2017-06-16
中文翻译:

通过非规范的萜类环化酶进行的吲哚羟化作用与细菌异源生物的解毒作用平行。
萜类天然产物包含多种分子结构,通常由经典I / II型萜烯环化酶催化的CC键形成所致。然而,完全不相关的,非规范的萜类环化酶大大提高了生物活性萜类的分子多样性。它们的进化起源一直是谜。在这里,我们报告了不寻常的黄素依赖性细菌吲哚萜类化合物环化酶XiaF,以及指定的黄素酶还原酶(XiaP)的体外重组,该酶介导了烟霉素生物合成的关键步骤。结合FADH 2的XiaF的晶体结构(在2.4Å分辨率下)和系统发育分析表明,XiaF与异源降解酶最密切相关。生物转化试验表明,XiaF是一种指定的吲哚羟化酶,可用于生产靛蓝和靛玉红。我们揭示了一种隐蔽的羟基化步骤,该步骤为萜类化合物环化奠定了基础,并表明环化酶已从异源生物解毒酶演变而来。

































 京公网安备 11010802027423号
京公网安备 11010802027423号