当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Efficient and Stereoselective Syntheses of Isomerically Pure 4-Aminotetrahydro-2H-thiopyran 1-Oxide Derivatives
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2017-06-15 00:00:00 , DOI: 10.1021/acs.oprd.7b00147 Ryo Mizojiri 1 , Kazuaki Takami 1 , Tatsuya Ito 2 , Hiroyuki Maeda 2 , Mitsuhisa Yamano 2 , Tetsuji Kawamoto 1
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2017-06-15 00:00:00 , DOI: 10.1021/acs.oprd.7b00147 Ryo Mizojiri 1 , Kazuaki Takami 1 , Tatsuya Ito 2 , Hiroyuki Maeda 2 , Mitsuhisa Yamano 2 , Tetsuji Kawamoto 1
Affiliation
|
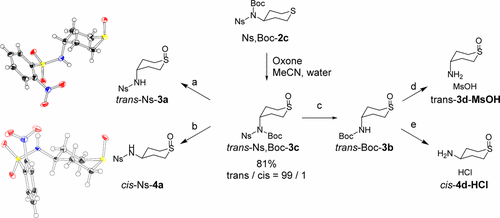
Efficient and stereoselective syntheses of isomerically pure 4-aminotetrahydro-2H-thiopyran 1-oxide derivatives have successfully been achieved. Isomerically pure (4-nitrophenyl)sulfonyltetrahydro-2H-thiopyran 1-oxides were identified by X-ray crystallographic analyses, and isomerically pure sulfoxide derivatives were characterized by 1H NMR. An oxidation reaction of tert-butyl(4-nitrophenyl)sulfonyl(tetrahydro-2H-thiopyran-4-yl)carbamate with Oxone provided steric control, affording its trans sulfoxide with high efficiency and selectivity. From the obtained trans sulfoxide derivatives, cis sulfoxide derivatives were synthesized conveniently by a hydrogen chloride catalyzed isomerization.
中文翻译:

异构纯4-氨基四氢-2 H-噻喃-1-氧化物衍生物的高效立体选择性合成
已成功实现了异构体纯的4-氨基四氢-2 H-硫代吡喃1-氧化物衍生物的高效和立体选择性合成。通过X射线晶体学分析鉴定了异构体纯的(4-硝基苯基)磺酰基四氢-2 H-噻喃1-氧化物,并且通过1 H NMR表征了异构体纯的亚砜衍生物。叔丁基(4-硝基苯基)磺酰基(四氢-2 H-硫代吡喃-4-基)氨基甲酸酯与氧酮的氧化反应提供了空间控制,从而提供了高效率和选择性的反式亚砜。由所获得的反式亚砜衍生物,通过氯化氢催化的异构化方便地合成了顺式亚砜衍生物。
更新日期:2017-06-28
中文翻译:

异构纯4-氨基四氢-2 H-噻喃-1-氧化物衍生物的高效立体选择性合成
已成功实现了异构体纯的4-氨基四氢-2 H-硫代吡喃1-氧化物衍生物的高效和立体选择性合成。通过X射线晶体学分析鉴定了异构体纯的(4-硝基苯基)磺酰基四氢-2 H-噻喃1-氧化物,并且通过1 H NMR表征了异构体纯的亚砜衍生物。叔丁基(4-硝基苯基)磺酰基(四氢-2 H-硫代吡喃-4-基)氨基甲酸酯与氧酮的氧化反应提供了空间控制,从而提供了高效率和选择性的反式亚砜。由所获得的反式亚砜衍生物,通过氯化氢催化的异构化方便地合成了顺式亚砜衍生物。






























 京公网安备 11010802027423号
京公网安备 11010802027423号