当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Estimation of the Thermochemical Radii and Ionic Volumes of Complex Ions
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2017-06-14 00:00:00 , DOI: 10.1021/acs.inorgchem.7b01205 Marcus C. Simoes 1 , Kevin J. Hughes 1 , Derek B. Ingham 1 , Lin Ma 1 , Mohamed Pourkashanian 1
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2017-06-14 00:00:00 , DOI: 10.1021/acs.inorgchem.7b01205 Marcus C. Simoes 1 , Kevin J. Hughes 1 , Derek B. Ingham 1 , Lin Ma 1 , Mohamed Pourkashanian 1
Affiliation
|
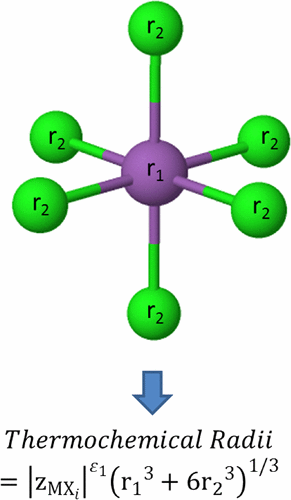
The estimation of the thermochemical radius is very important because most of the properties of the electrolyte solutions are, to some extent, linked to this property. Also, these thermochemical radii can be used to estimate lattice energies, which can be a very important parameter to be evaluated when assessing the possibility of synthesizing new inorganic materials. This study presents a formulation for estimating the thermochemical radii of complex ions. More specifically, these thermochemical radii are estimated using a weighted sum based on the radii of the contributing cations and anions. Also, the influence of the ionic charge on these thermochemical radii is assessed and discussed. Finally, the parameters obtained from the estimation of the thermochemical radii of complex cations are used to estimate cation volumes, and this estimation is then validated through comparison with literature values. As a result, the equations developed for thermochemical radii of complex ions produce predictions that are accurate to within 15% in general, whereas the equation developed to estimate cation volumes produces predictions that are accurate to within 20% considering cation volumes greater than 70 Å3.
中文翻译:

估算复杂离子的热化学半径和离子体积
热化学半径的估计非常重要,因为电解质溶液的大多数性质在某种程度上与该性质有关。同样,这些热化学半径可用于估计晶格能量,当评估合成新的无机材料的可能性时,晶格能量是非常重要的参数。这项研究提出了一种估算复合离子热化学半径的公式。更具体地,这些热化学半径是基于所贡献的阳离子和阴离子的半径而使用加权和来估计的。同样,评估并讨论了离子电荷对这些热化学半径的影响。最后,将从复杂阳离子的热化学半径估算中获得的参数用于估算阳离子体积,然后通过与文献值进行比较来验证该估计。结果,为复杂离子的热化学半径而开发的方程式通常可得出准确度在15%以内的预测,而为估计阳离子量而开发的方程式可在阳离子量大于70Å时得出准确度在20%以内的预测。3。
更新日期:2017-06-14
中文翻译:

估算复杂离子的热化学半径和离子体积
热化学半径的估计非常重要,因为电解质溶液的大多数性质在某种程度上与该性质有关。同样,这些热化学半径可用于估计晶格能量,当评估合成新的无机材料的可能性时,晶格能量是非常重要的参数。这项研究提出了一种估算复合离子热化学半径的公式。更具体地,这些热化学半径是基于所贡献的阳离子和阴离子的半径而使用加权和来估计的。同样,评估并讨论了离子电荷对这些热化学半径的影响。最后,将从复杂阳离子的热化学半径估算中获得的参数用于估算阳离子体积,然后通过与文献值进行比较来验证该估计。结果,为复杂离子的热化学半径而开发的方程式通常可得出准确度在15%以内的预测,而为估计阳离子量而开发的方程式可在阳离子量大于70Å时得出准确度在20%以内的预测。3。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号