Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2017-06-13 , DOI: 10.1016/j.bmc.2017.06.014 Daisuke Matsuda , Yohei Kobashi , Ayako Mikami , Madoka Kawamura , Fumiyasu Shiozawa , Kenichi Kawabe , Makoto Hamada , Shinichi Nishimoto , Kayo Kimura , Masako Miyoshi , Noriko Takayama , Hiroyuki Kakinuma , Norikazu Ohtake
|
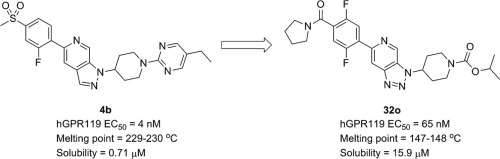
We previously reported a novel series of 1H-pyrazolo[3,4-c]pyridine derivatives and the identification of compound 4b as a highly potent GPR119 agonist. However, the advancement of preclinical evaluations of compound 4b is expected to be difficult because of the compound’s significantly poor aqueous solubility (0.71 μM at pH6.8). In this article, we describe the further optimization of compound 4b focusing on the improvement of its aqueous solubility. Optimization of the central spacer, left-hand aryl group and right-hand piperidine N-capping group led to the identification of a potent GPR119 agonist, 3H-[1,2,3]triazolo[4,5-c]pyridine derivative 32o, with improved solubility (15.9 μM at pH6.8).
中文翻译:

新型3 H- [1,2,3]三唑并[4,5- c ]吡啶衍生物作为GPR119激动剂:合成与结构-活性/溶解度关系
我们先前报道了一系列新型的1 H-吡唑并[3,4- c ]吡啶衍生物,并将化合物4b鉴定为高效GPR119激动剂。但是,由于该化合物的水溶性差(在pH6.8下为0.71μM),因此很难进行化合物4b的临床前评估。在本文中,我们描述了化合物4b的进一步优化,重点是提高其水溶性。优化中央间隔基,左手的芳基和右手的哌啶N-封端基团导致鉴定出强效的GPR119激动剂3 H- [1,2,3]三唑[4,5- c ]]吡啶衍生物32o,具有改善的溶解度(在pH6.8下为15.9μM)。






























 京公网安备 11010802027423号
京公网安备 11010802027423号