当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Extreme Dynamics in the BamA β-Barrel Seam
Biochemistry ( IF 2.9 ) Pub Date : 2017-06-12 00:00:00 , DOI: 10.1021/acs.biochem.7b00281 Pamela Arden Doerner 1 , Marcelo C. Sousa 1
Biochemistry ( IF 2.9 ) Pub Date : 2017-06-12 00:00:00 , DOI: 10.1021/acs.biochem.7b00281 Pamela Arden Doerner 1 , Marcelo C. Sousa 1
Affiliation
|
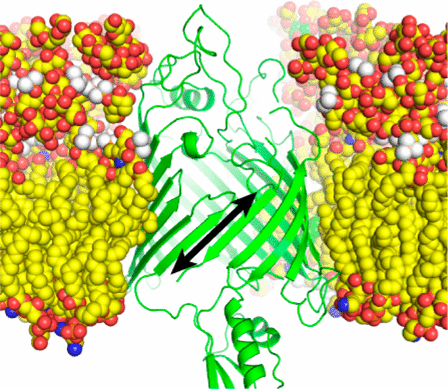
BamA is an essential component of the β-barrel assembly machine (BAM) that is responsible for insertion and folding of β-barrel outer membrane proteins (OMPs) in Gram-negative bacteria. BamA is an OMP itself, and its β-barrel transmembrane domain is thought to catalyze OMP insertion and folding, although the molecular mechanism remains poorly understood. Crystal structures of BamA and complementary molecular dynamics simulations have shown that its β-barrel seam (the interface between the first and last barrel strands) is destabilized. This has led to mechanistic models in which the BamA barrel seam functions as a lateral gate that opens and successively accepts β-hairpins from a nascent OMP such that a nascent barrel can bud from BamA. Consistent with this model, disulfide locking of the BamA barrel seam is lethal in Escherichia coli. Here we show that disulfide locking of the BamA barrel has no effect on its ability to catalyze folding of a model OMP into liposomes. However, disulfide trapping experiments indicate that the BamA barrel is highly dynamic in the liposome membranes, with the β-strands at the barrel seam undergoing “register sliding” by more than 14 Å both up and down the membrane. Remarkably, these extreme dynamics were also observed in the BamA barrel in the context of the native E. coli outer membrane. These results are consistent with a model in which the BamA barrel dynamics induce defects in the outer membrane that facilitate insertion of nascent OMPs.
中文翻译:

BamAβ桶接缝中的极端动力学
BamA是β桶装配机器(BAM)的重要组成部分,负责在革兰氏阴性细菌中插入和折叠β桶外膜蛋白(OMP)。BamA本身是OMP,尽管其分子机理尚不清楚,但其β-桶状跨膜结构域被认为可催化OMP的插入和折叠。BamA的晶体结构和互补的分子动力学模拟表明,其β桶接缝(第一和最后一桶链之间的界面)不稳定。这导致了一种机械模型,其中BamA枪管接缝用作侧门,该门打开并接连接受新生OMP的β发夹,以便新生枪管可以从BamA发芽。与该模型一致,BamA桶接缝的二硫化物锁定在大肠杆菌。在这里,我们显示BamA桶的二硫键锁定对其催化模型OMP折叠成脂质体的能力没有影响。但是,二硫键捕获实验表明,BamA桶在脂质体膜中具有很高的动态性,桶缝处的β链在膜上和下都经历“套准滑动”超过14Å。值得注意的是,在天然大肠杆菌外膜的背景下,在BamA桶中也观察到了这些极端动态。这些结果与其中BamA桶动力学在外膜中引起有助于新生OMP插入的缺陷的模型一致。
更新日期:2017-06-13
中文翻译:

BamAβ桶接缝中的极端动力学
BamA是β桶装配机器(BAM)的重要组成部分,负责在革兰氏阴性细菌中插入和折叠β桶外膜蛋白(OMP)。BamA本身是OMP,尽管其分子机理尚不清楚,但其β-桶状跨膜结构域被认为可催化OMP的插入和折叠。BamA的晶体结构和互补的分子动力学模拟表明,其β桶接缝(第一和最后一桶链之间的界面)不稳定。这导致了一种机械模型,其中BamA枪管接缝用作侧门,该门打开并接连接受新生OMP的β发夹,以便新生枪管可以从BamA发芽。与该模型一致,BamA桶接缝的二硫化物锁定在大肠杆菌。在这里,我们显示BamA桶的二硫键锁定对其催化模型OMP折叠成脂质体的能力没有影响。但是,二硫键捕获实验表明,BamA桶在脂质体膜中具有很高的动态性,桶缝处的β链在膜上和下都经历“套准滑动”超过14Å。值得注意的是,在天然大肠杆菌外膜的背景下,在BamA桶中也观察到了这些极端动态。这些结果与其中BamA桶动力学在外膜中引起有助于新生OMP插入的缺陷的模型一致。

































 京公网安备 11010802027423号
京公网安备 11010802027423号