Chemical Engineering Journal ( IF 13.3 ) Pub Date : 2017-05-04 , DOI: 10.1016/j.cej.2017.05.005 V.I. Simagina , N.V. Vernikovskaya , O.V. Komova , N.L. Kayl , O.V. Netskina , G.V. Odegova
|
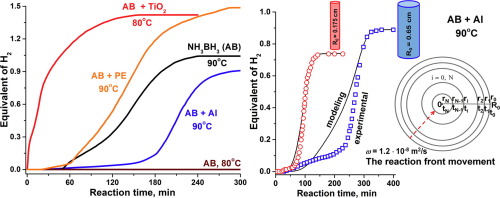
Ammonia borane (NH3BH3, AB) is considered to be a promising hydrogen storage material owing to its very high content of hydrogen (19.6 wt%), high stability in air at ambient temperatures and the low temperature of the dehydrogenation process. In this work solid-state decomposition of NH3BH3 in contact with a series of solid materials has been investigated. It was shown that the reactivity of the studied AB-based hydrogen-generating systems was changing under the action of both the chemical nature and thermal conducting properties of the studied modifiers. It is important that, according to ATR-FTIR spectroscopy, the contact of AB with oxygen-containing supports (TiO2, γ-Al2O3, SiO2, MgO, HY zeolite) destabilizes the AB structure to evolve hydrogen already at 80 °C, independently of their chemical nature. On the other hand, it was shown that in a heat insulator reaction medium the temperature in the reaction zone increases leading to an increased yield of hydrogen. In addition to this, the reaction properties of AB have for the first time been studied depending on the radius of the tubular reactor during the low-temperature dehydrogenation (90 °C) under conditions preventing appearance of local thermal spikes. A mathematical model has been developed which describes the obtained experimental results taking into account the propagation of the reagent-product interface from the heated reactor wall towards its axis.
中文翻译:

氨硼烷储氢系统的实验与建模研究
氨硼烷(NH 3 BH 3,AB)被认为是一种有前途的储氢材料,因为其氢含量很高(19.6 wt%),在环境温度下在空气中具有很高的稳定性以及脱氢过程的低温。在这项工作中,已经研究了与一系列固体材料接触时NH 3 BH 3的固态分解。结果表明,所研究的基于AB的制氢系统的反应性在所研究的改性剂的化学性质和导热性质的作用下均发生了变化。这是重要的,根据ATR-FTIR光谱学,AB的二氧化钛的接触含氧支撑件(2,γ-Al系2 ö如图3所示,SiO 2,MgO,HY沸石使AB结构不稳定并在80°C时已经析出氢,而与它们的化学性质无关。另一方面,已经表明,在绝热反应介质中,反应区中的温度升高,导致氢气产率提高。除此之外,在低温脱氢(90°C)期间,在防止出现局部热尖峰的条件下,首次根据管式反应器的半径研究了AB的反应特性。已经开发了数学模型,该模型描述了考虑到试剂-产物界面从加热的反应器壁朝向其轴的传播的获得的实验结果。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号