Tetrahedron ( IF 2.1 ) Pub Date : 2017-06-10 , DOI: 10.1016/j.tet.2017.06.001 Puli Saidhareddy , Sama Ajay , Arun K. Shaw
|
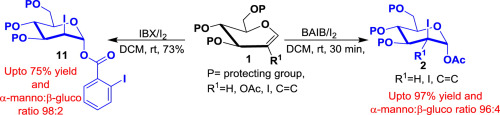
Two efficient, metal free reagent systems, PhI(OAc)2-I2 (method A) and IBX-I2 (method B), for stereoselective synthesis of trans-2-deoxy-2-iodoglycosylacetates and O-iodobenzoates respectively from differently protected glycals have been developed. They are compatible with a variety of protecting groups and various functional groups at 2C-position. Hexose-3,2-enolone 8 is obtained directly from 2-acetoxy glycal 5 by method A. An application to modified method B has been shown by synthesis of a diastereomerically pure α-glycosyl ortho-hexynylbenzoate 12, a glycosyl donor from 3,4,6-tri-O-acetyl-D-glucal in two steps that has been further utilized in the synthesis of glycosides 13–18.
中文翻译:

碘代二苯二乙酸碘和IBX碘:从受保护的糖中合成非对映异构体富集的乙酸2-脱氧-2-碘糖基乙酸酯和2-脱氧-2-碘基糖基邻碘苯甲酸酯的试剂系统
两种有效的无金属试剂系统PhI(OAc)2 -I 2(方法A)和IBX-I 2(方法B),分别用于立体选择性地合成反式-2-脱氧-2-碘代糖基乙酸酯和O-碘代苯甲酸酯已经开发了保护的糖基。它们在2 C位与各种保护基和各种官能团相容。通过方法A直接从2-乙酰氧基乙二醇5获得己糖3,2-烯醇酮8。通过合成非对映体纯的α-糖基邻-己炔基苯甲酸酯12显示了对改良方法B的应用。从3,4,6-三糖供体ö乙酰基d-己烯糖在已在糖苷的合成被进一步利用两个步骤13 - 18。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号